Sterilization Methods and Apparatus which Employ Additive-Containing Supercritical Carbon Dioxide Sterilant
Sterilization Methods and Apparatus which Employ Additive-Containing Supercritical Carbon Dioxide Sterilant
US7108832
Company:
Year:
Abstract:
Sterilization methods and apparatus are effective to achieve a 6-log reduction in CFUs of industry standard bacteria and bacterial spores, i.e., B. stearothermophilus and B. subtilis spores, by subjecting sterilizable materials to a chemical additive-containing carbon dioxide sterilant fluid at or near its supercritical pressure and temperature conditions. Most preferably, the chemical additive-containing supercritical carbon dioxide sterilant fluid is agitated during sterilization, e.g., via mechanical agitation or via pressure cycling.
Type of document:
Language:
United States Patent
US007l08832B2
(12) (10) Patent No.: US 7,108,832 B2
Christensen et al. (45) Date of Patent: Sep. 19, 2006
(54) STERIALIZATION METHODS AND 5,851,483 A * 12/1998 Nicolle et al. .............. .. 422/28
APPARATUS WHICH EMPLOY 5,996,155 A * 12/1999 Chao et al. .................. .. 8/158
é§3E(§;7E];€(()’§}]T)1EI§ITPl::(l}{ISIJIi1;1E]13CRITICAL 6,149,864 A 11/2000 Dillow et al.
6,518,307 B1* 2/2003 McKenzie et al. ........ .. 514/557
(75) Inventors: Timothy Wayne Christensen, Ithaca, 6’613’278 B1 9/2003 Mills et 31'
NY (US); David Carroll Burns, Ithaca, 6,716,457 B1 * 4/2004 Eagles et al. ............. .. 424/616
NY (US); Angela Lydia White, 2003/0072677 A1 4/2003 Kafesjian et al.
Truxton, NY (US); Bruce Ganem, 2004/0033269 A1* 2/2004 Hei et al. ................. .. 424/616
Ithaca, NY (US);Anth0I1y Rome)’ 2004/0120852 A1 6/2004 Karmo
Eisenhut, Lansing, NY (US)
(73) Assignee: NoVaSterilis Inc., Ithaca, NY (US)
OTHER PUBLICATIONS
( * ) Notice: Subject to any disclaimer, the term of this
patent is extended Or adjusted under 35 Holyoak et al, “Toxic effects of ethylene oxide residuals on bovine
U.S,C, 154(b) by 0 days, embryos in vitro”, TOXICOLOGY, 108, 1996, pp. 33-38.
Cornu et al, “Effect of Freeze-Drying and Gamma Irradiation on the
(21) Appl. No.: 10/869,052 Mechanical Properties of Human Cancellous Bone”, Journal of
Orthopaedic Research, vol. 18, No. 3, 2000, pp. 426-431.
(22) Filed J“n- 17: 2004 Spilimberrgo et al, “Microbial inactivation by high-pressure”, Jour-
nal of Supercritical Fluids, 22, 2002, pp. 55-63.
(65) Prlor Pubhcatlon Data Akkus et al, “Fracture resistance of gamma radiation sterilized
Us 2005/0025667 A1 Feb. 3, 2005 :((;i(t11caIl)pb(;I;e7_gl3l:grafts”, Journal of Orthopaedic Research, 19,
Related U.S. Application Data (Continued)
(60) :’go\éi(s)i0o3nal application No. 60/480,410, filed on Jun. Primary Examl.neriKI_iSanne Jastrzab
’ ' (74) Attorney, Agent, or F irm—Welsh & Flaxman LLC
(51) Int. Cl.
A61L 2/00 (2006.01) (57) ABSTRACT
(52) U.S. Cl. .......................... .. 422/28; 422/28; 422/31;
_ _ 22/33; 422/119; 422/297; 516/9 Sterilization methods and apparatus are effective to achieve
(58) Field of Classification Search .................. .. 134/1; a 6_10g reductionin CFUS Ofindustry Standard bacteria and
204/15715’ 1582; 422/42:2’ bacterial spores, i.e., B. stearothermophilus and B. subtilis
S 1. . fil f 1 h h. ’ spores, by subjecting sterilizable materials to a chemical
ee app lcanon e or Comp ete Seam lstory‘ additive-containing carbon dioxide sterilant fluid at or near
(56) References Cited its supercritical pressure and temperature conditions. Most
U.S. PATENT DOCUMENTS
4,944,837 A * 7/1990 Nishikawa et al. ......... .. 216/41
5,213,619 A * 5/1993 Jackson et al. . . . . . . . . .. 134/1
5,370,740 A * 12/1994 Chao et al. .................. .. 134/1
preferably, the chemical additive-containing supercritical
carbon dioxide sterilant fluid is agitated during sterilization,
e.g., via mechanical agitation or via pressure cycling.
9 Claims, 2 Drawing Sheets
- H
PFESSUFB
VESSEI
US 7,108,832 B2
Page 2
OTHER PUBLICATIONS
Jahan et al, “Long-Term Effects of Gamma-Sterialization on Deg-
radation of Implant Materials”, Appl. Radiat. Isot., Vol. 46, No. 6/7,
1995, pp. 637-638.
Ikarashi et al, “Cytotoxicity of medical materials sterilized with
Vapour-phase hydrogen peroxide”, BIOMATERIALS, Vol. 16, No.
3, 1995, pp. 177-183.
Duffy et al, “An Epidemic of Corneal Destruction Caused by
Plasma Gas Sterilization”, Arch. Ophthalmol., Vol. 118, Sep. 2000,
pp. 1167-1176.
Godette et al, “Biomechanical Effects of Gamma Irradiation on
Fresh Frozen Allografts in Vivo”, ORTHOPEDICS, Vol. 19, No. 8,
Aug. 1996, pp. 649-653.
Schiewe et al, “Toxicity Potential of Absorbed-Retained Ethylene
Oxide Residues in Culture Dishes on Embryo Development in
Vitro”, Journal of Animal Science, Vol. 60, No. 6, 1985, pp.
1610-1618.
Windebar1k et al, “Residual Ethylene Oxide in Hollow Fiber
Hemodialysis Units Is Neurotoxic in Vitro”, Annals of Neurology,
Vol. 26, No. 1, Jul. 1989, pp. 63-68.
* cited by examiner
N ma
E
U.S. Patent Sep. 19,2006 Sheet 1 of2 US 7,108,832 B2
3
_$8>
mm
8 8
o>_m>
R 3
_ wa
smaaeoo
__<
E
_%=__>o
N8
@
S
U.S. Patent Sep. 19,2006 Sheet 2 of2 US 7,108,832 B2
6
U) 5
E
C.)
.5
.5 4
*5’
§
‘E. 3
.4 Regression
2 "" “ 95% CI
20 30 40 50 60
Time; Minutes
Fig. 3
US 7,108,832 B2
1
STERIALIZATION METHODS AND
APPARATUS WHICH EMPLOY
ADDITIVE-CONTAINING SUPERCRITICAL
CARBON DIOXIDE STERILANT
CROSS REFERENCE TO RELATED
APPLICATION
This application is based on, and claims domestic priority
benefits under 35 U.S.C. §119(e) from, Provisional Appli-
cation No. 60/480,410, filed Jun. 23, 2003, the entire content
of which is hereby incorporated by reference.
FIELD OF THE INVENTION
The present invention relates generally to sterilization
methods and apparatus in which supercritical carbon dioxide
is employed as a sterilization fluid. In especially preferred
embodiments, the present invention relates to methods and
apparatus in which the eflicacy of the supercritical carbon
dioxide is enhanced by certain chemical additives.
BACKGROUND OF THE INVENTION
A need has developed in the tissue implantation or trans-
plantation, biomedical polymers, medical equipment, and
drug delivery industries for a gentle and reliable sterilization
method that results in greater than 106 log reductions of
microbial and viral contaminants without impacting the
properties of the material being sterilized. Indeed many new
medical advances carmot be implemented because the ster-
ilization industry is unable to provide a suitable sterilant as
part of the manufacturing process.
In the case of polymers, gamma irradiation has been
shown to compromise the mechanical properties.1 Further-
more, steam sterilization is incompatible with thermally or
hydrolytically labile polymers. Ethylene oxide, a common
and widely used sterilant, is toxic, mutagemc, and a carci-
nogenic substance that can react with some polymers, and
also requires prolonged periods of outgassing.
llahan et al, “Long-term effects of gamma-sterilization on degradation of
implant materials.” Applied Radiation and Isolopes: Including Dala, Inslru-
menzarion and Mezhods For Use in Agricullure, Induslry and Medicine
46(6—7): 637—8 (1995), incorporated expressly hereinto by reference.
Biological tissues, including macromolecular biopoly-
mers, are also incompatible with steam. Gamma radiation
results in a significant decrease in tissue integrity and bone
strength.2 Certain antibacterial washes have been used to
disinfect tissue, but incomplete sterilization is achieved and
the washes leave residual toxic contaminants in the tissues.3
Ethylene oxide also reacts with biological tissue and is thus
an undesirable sterilant for such reason.
2 Comu et al, “Eflect of freeze-drying and gamma irradiation on the
mechanical properties of human caucellous bone”, Joumal of Orthopaedic
Research, 18(3), p. 426-31 (2000); and Akkus et al, “Fracture resistance of
gamma radiation sterilized cortical bone allografts.” Journal of Orlhopaedic
Research: Ofiicial Publicalion of lhe Orlhopaedic Research Sociely 19(5):
927—34 (2001), the entire content of each incorporated expressly hereinto by
reference.
3Holyoak et al, “Toxic effects of ethylene oxide residues on bovine embryos
in vitro”, Toxicology, 108(1—2, p. 33—8 (1996), the entire content of each
incorporated hereinto by reference.
Many medical devices, such as stents, catheters and
endoscopes, are fabricated from, or coated with, sensitive
polymers that cannot tolerate steam, irradiation, or ethylene
oxide. Plasma sterilization has been shown to be incompat-
ible with some medical equipment and leaves toxic residues
(Ikarashi, Tsuchiya et al. 1995; Duffy, Brown et al. 2000).4
4Ikarashi et al, “Cytotoxicity of medical materials sterilized with vapour-
phase hydrogen peroxide.” Biomalerials 16(3): 177—83 (1995) and Duffy et
al, “An epidemic of comeal destruction caused by plasma gas sterilization.
10
15
20
25
30
35
40
45
50
55
60
65
2
The Toxic Cell Destruction Syndrome Investigative Team.” Archives of
Ophzhalmology 118(9): 1167—76 (2000), the entire content of each expressly
incorporated hereinto by reference.
Recently, in U.S. Pat. No. 6,149,864 to Dillow et al (the
entire content of which is expressly incorporated hereinto by
reference), the use of supercritical CO2 was disclosed as an
altemative to existing technologies for sterilizing a wide
range of products for the healthcare industry with little or no
adverse effects on the material treated.
Specifically, the Dillow ’864 patent disclosed the inacti-
vation of a wide range of vegetative microbial cells using
supercritical carbon dioxide with agitation and pressure
cycling. However, only one spore-forming bacterium was
investigated in the Dillow ’864 patent, specifically, B.
cereus. No disclosure appears in Dillow ’864 patent regard-
ing the eflicacy of the therein suggested techniques using
currently accepted bio-indicator standards used to judge
sterilization (i.e., B. slearozhermophilus and B. subtilis).
Subsequently, however, other investigators achieved only a
3.5 log reduction in B. sublilis spores using the method
disclosed in the Dillow et al ’864 patent.5
5Spilimbergo et al, “Microbial inactivation by high-pressure.” J. Supercrirical
Fluids 22: 55—63 (2002), the entire content expressly incorporated hereinto by
reference.
Bacterial spores are more diflicult to sterilize than veg-
etative cells. B. szearothermophilus and B. subtilis spores
represent the greatest challenge to sterilization methods
(FDA 1993) and are the currently accepted standards within
the industry for validating sterilization methods. Steriliza-
tion is defined as greater than or equal to 6-log (106)
reduction in colony forming units (CFUs). Reproducible
inactivation of these resistant microbes is required for com-
mercialization of novel sterilization equipment and pro-
cesses.
It therefore would be highly desirable if sterilization
methods and apparatus could be provided which are effec-
tive to achieve a 6-log reduction in CFUs of industry
standard bacterial spores. It would more specifically be
especially desirable if sterilization methods and apparatus
could be provided that achieve a 6-log reduction in CFUs of
B. slearothermophilus and B. subtilis spores. The present
invention is therefore directed to fulfilling such needs.
SUMMARY OF THE INVENTION
Broadly, sterilization methods and apparatus are provided
by the present invention which are effective to achieve a
6-log reduction in CFUs of industry standard bacterial
spores. More specifically, according to the present invention,
sterilization methods and apparatus are provided which are
effective to achieve a 6-log reduction in CFUs of B. steam-
thermophilus and B. sublilis spores. These 6-log reductions
are achieved by the present invention by subjecting steril-
izable materials under sterilization pres sure and temperature
conditions using a chemical additive-containing supercriti-
cal carbon dioxide as a sterilant fluid. Most preferably, the
chemical additive-containing supercritical carbon dioxide
sterilant fluid is agitated during sterilization.
The apparatus and methods of the present invention are
especially well suited for the sterilization of thermally or
hydrolytically sensitive, medically-important materials,
including biodegradable and other medical polymers, tissue
for implantation or transplantation, medical equipment,
drugs and drug delivery systems. Most preferably, such
materials are sterilized by treatment with a chemical addi
US 7,108,832 B2
3
tive-containing carbon dioxide sterilant at or near its super-
critical pressures and temperatures.
Sterilization is specifically further enhanced by imparting
turbulence or agitation to the sterilant fluid either mechani-
cally or by means of pressure cycling (see, the above-cited
Dillow et al ’864 patent). Process variables depend on the
material being sterilized. The improved method enhances
the mass transfer and sterilization capabilities of supercriti-
cal carbon dioxide. Medically useful log reductions (>106)
in microbial contaminants are realized for a range of resis-
tant bacteria, their Vegetative forms, and spores, especially
bacteria and bacterial spores which are traditionally known
to be the hardest to inactivate, such as B. slearozheromophi—
lus, B. pumilus and/or B. subtilis and spores. Thus, as used
herein the term “sterilization” is meant to refer to at least a
6-log (>106) reduction of industry standard bacteria and
related bacterial spores selected from B. slearozheromophzl
lus, B. pumilus and/ or B. subtilis. Thus, a “sterile” surface or
article is one which has at least a 6-log (>106) reduction of
such bacteria and spores following a sterilization treatment,
as compared to the surface or article prior to such steriliza-
tion treatment.
These and other aspects and advantages will become more
apparent after careful consideration is given to the following
detailed description of the preferred exemplary embodi-
ments thereof.
BRIEF DESCRIPTION OF THE
ACCOMPANYING DRAWINGS
Reference will hereinafter be made to the accompanying
drawings, wherein like reference numerals throughout the
various FIGURES denote like structural elements, and
wherein;
FIG. 1 is a schematic view of a presently preferred
sterilization apparatus in accordance with the present inven-
tion;
FIG. 2 is a detailed schematic view of the pressure vessel
employed in the apparatus of FIG. 1; and
FIG. 3 is a graph of the log reduction in CFU’s of B.
slearothermophilus spores versus time obtained from the
data of Example 8 below and shows the linearity of inacti-
vation achieved by means of the present invention.
DETAILED DESCRIPTION OF THE
INVENTION
The sterilization apparatus and methods of the present
invention are usefully employed to sterilize a variety of
materials, biological tissues, instruments, and devices that
are thermally or hydrolytically unstable, or otherwise
incompatible with conventional sterilization techniques, or
where such techniques are not preferred. Examples of mate-
rials that may be sterilized by the present invention include,
but are not limited to, biodegradable polymers such as
poly(lactic acid) (PLA) or poly(lactic-co-glycolic acid)
(PLGA)-based polymers, which can be used in various
embodiments as implantable drug delivery devices; tissues
for implantation or transplantation, including but not limited
to, bone, cartilage, ligament, or other connective or muscu-
loskeletal tissue for allografts in the treatment of orthopaedic
trauma and joint reconstruction; grafted or artificial skin
tissue for the treatment of bums and other dermal abrasions
or damage; medical devices, such as cardiac or urological
stents and catheters, including drug- or gene-coated stents
and catheters, rigid and flexible endoscopes for orthopaedic,
plastic, and gastroenterological surgery; drug delivery
10
15
20
25
30
35
40
45
50
55
60
65
4
devices, including, but not limited to, implantable polymer
devices, polymer microspheres, or other specifically shaped
drug-releasing devices comprised of PLA, PLGA, or other
biodegradable polymers, and drugs in solid or liquid forms
(i.e., any substance or active agent used in the diagnosis,
treatment or prevention of a disease or illness).
As noted previously, 6-log reductions in CFUs may be
achieved in accordance with the present invention by sub-
jecting materials to be sterilized under sterilization tempera-
ture and pressure conditions using a chemical additive-
containing supercritical carbon dioxide as a sterilant fluid,
and especially where the sterilant fluid is agitated during the
sterilization process.
Most preferably, the sterilant is carbon dioxide at or near
its supercritical pressures and temperature conditions. Thus,
the sterilization process of the present invention is practiced
using carbon dioxide as a sterilant at pressures between
about 1000 to about 3500 psi, at temperatures in the range
between about 25° C. to about 60° C. Most preferably, the
article to be sterilized is subject to carbon dioxide at or near
such pressure and temperature conditions for times ranging
from about 20 minutes to about 12 hours. The carbon
dioxide employed in the practice of the present invention is
most preferably substantially pure. Thus, trace amounts of
other gases may be tolerated provided that the sterilization
properties of the carbon dioxide are not impaired. For ease
of further discussion below, the term “supercritical carbon
dioxide” will be used, but it will be understood that such a
term is non-limiting in that carbon dioxide within the
pressure and temperature ranges as noted immediately above
may be employed satisfactorily in the practice of the present
invention.
The chemical additives employed in the present invention
most preferably include peroxides and/or carboxylic acids.
Preferred carboxylic acids include alkanecarboxylic acids
and/or alkanepercarboxylic acids, each of which may
optionally be substituted at the alpha carbon with one or
more electron-withdrawing substituents, such as halogen,
oxygen and nitrogen groups. Particularly preferred species
of chemical additives employed in the practice of the present
invention include hydrogen peroxide (H202), acetic acid
(AcA), peracetic acid (PAA) and trifluoroacetic acid (TFA),
and mixtures thereof. One particularly preferred liquid addi-
tive that may be employed in the practice of the present
invention is commercially available Sporeclenz® sterilant
which is a mixture of acetic acid with hydrogen peroxide and
peracetic acid.
The chemical sterilization additive is employed in a
sterilization enhancing effective amount of at least about
0.001 vol. % and greater, based on the total volume of the
carbon dioxide. The amount of sterilization additive will be
dependent upon the particular sterilization additive that is
employed. Thus, for example, peracetic acid may be present
in relatively small amounts of about 0.005 vol. % and
greater, while acetic acid may need to be employed in
amount of about 1.0 vol. % and greater. Thus, a range of at
least about 0.001 vol. % and greater, up to about 2.0 vol. %
will typically be needed in order to achieve a sterilization
enhancing effect in combination with carbon dioxide.
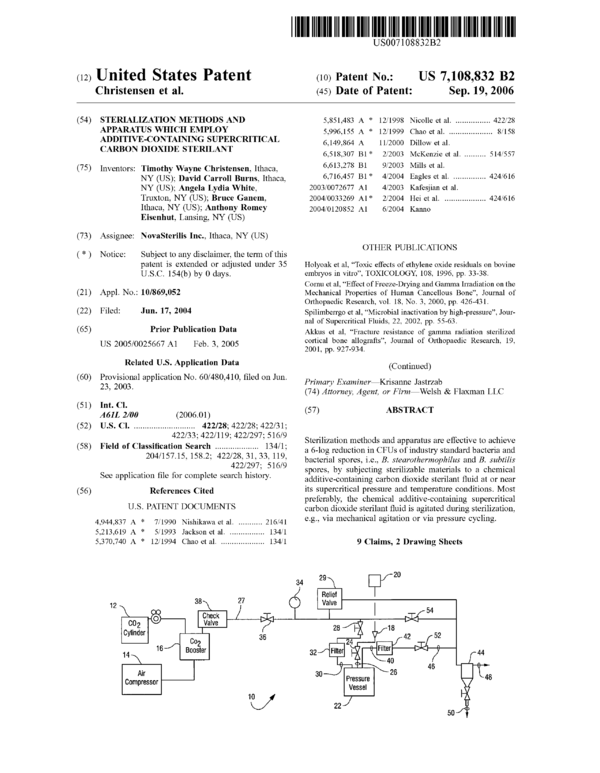
One presently preferred embodiment of an apparatus 10
according to the present invention is depicted in accompa-
nying FIGS. 1 and 2. In this regard, it can be seen that the
apparatus includes a standard compressed gas cylinder 12
containing carbon dioxide, and a standard air compressor 14
used in operative association with a carbon dioxide booster
US 7,108,832 B2
5
16 (e.g., Haskel Booster AGT 7/30). Alternatively, the air
compressor 14 and booster 16 can be replaced with a single
carbon dioxide compressor.
An additive cycle is also provided by means of a series of
an inlet port 18 which allows additive contained in reservoir
20 to be added to a pressure vessel 22 through valve 24 and
additive line 26. The carbon dioxide is introduced to the
pressure vessel 22 from header line 27 via valve 28 and CO2
supply line 30. A filter 32 (e.g., a 0.5 micron filter) is
provided in the supply line 30 to prevent escape of material
from the vessel. A pressure gauge 34 is provided down-
stream of CO2 shut-olf valve 36 in supply header 27 to allow
the pressure to be visually monitored. A check valve 38 is
provided in the line 27 upstream of the valve 36 to prevent
reverse fluid flow into the booster 16. In order to prevent an
overpressure condition existing in line 27, a pressure relief
valve 9 may be provided.
An outlet line 40 through valve 52 allows the pressure
vessel 22 to be depressurized. In this regard, the depressur-
ized fluid exits the vessel 22 via line 40, is filtered by filter
unit 42 and then is directed to separator 44 where filtered
CO2 gas may be exhausted via line 48, and liquid additive
collected via line 50 for possible reuse. Valves 52, 54 may
be provided in lines 46 and 27, respectively, to allow fluid
isolation of upstream components.
The reactor vessel 22 is most preferably constructed of
stainless steel (e.g., 316 gauge stainless steel) and has a total
internal volume sufficient to accommodate the materials
being sterilized either on a laboratory or commercial scale.
For example, in laboratory studies, an internal volume of
600 mL (e.g., approximately 8 inches long by about 2.5
inches inside diameter) was deemed adequate As is perhaps
more clearly shown in FIG. 2, the pressure vessel 22
includes a vibrator 60, a temperature control unit 62, and a
mechanical stirring system most preferably comprised of an
impeller 64 and a magnetic driver 66. The reactor vessel 22
contains a conventional basket (not shown) which is also
preferably constructed of 316 gauge stainless steel. The
basket serves to hold the items to be sterilized as well as to
protect the impeller 64 and direct the sterilant fluid in a
predetermined marmer.
The reactor vessel 22 may be operated at a constant
pressure or under continual pressurization and depressuriza-
tion (pressure cycling) conditions without material losses
due to splashing or turbulence, and without contamination of
pressure lines via back diffusion. The valves 24, 28 and 52
allow the vessel 22 to be isolated and removed easily from
the other components of the apparatus 10. The top 68 of the
pressure vessel 22 may be removed when depressurized to
allow access to the vessel’s interior.
In use, the material to be sterilized is introduced into the
interior space of the pressure vessel 22 along with any initial
portion of liquid sterilization additive from reservoir 20. The
temperature control unit 62 is operated so as to set the
desired initial temperature for sterilization. The vessel 22
may then be pre-equilibrated with carbon dioxide from gas
cylinder 12 at atmospheric pressure, following which the
magnetic driver 66 is operated so as to activate the impeller
64. The pressure vessel 22 may thereafter be pressurized to
a desired pressure by introducing additional carbon dioxide
gas from cylinder 12 via the air compressor 14 linked to
booster 16.
In order to effect a pressure cycling of the vessel 22, an
amount of carbon dioxide may be released therefrom via
depressurization line by momentarily opening valve 52
sufficient to partially reduce pressure within the vessel 22.
5
10
15
20
25
45
50
55
60
65
6
Additive may be introduced into the vessel 22 for any given
pressure cycle by opening valve 24 which allows liquid
additive to flow from reservoir 20 into inlet port 18. It will
be understood that the sterilization additives may be intro-
duced prior to pressurization and/ or during pressure cycling.
Prior to pressurization, additives are introduced directly into
the reactor vessel 22 prior to sealing and/or via the additive
port 18. The sterilization additives are most preferably
introduced during the cycling stages by measured addition to
the additive port 18 at ambient pressures. The port 18 is
subsequently sealed and the additive chamber is pressurized
so that the additive may enter the reactor vessel 22 without
altering the internal pressure. The exact mechanism of
addition may be modified such that the process is more
eflicient and/or convenient.
Following additive introduction, the vessel 22 may be
repressurized to a desired pressure following introduction of
the liquid additive therein. Such depressurization/repressur-
ization with introduction of liquid additive may be repeated
for any number of cycles that may be desired. The cycle of
depressurization and repressurization as well as the intro-
duction of the carbon dioxide and liquid additive may be
automatically controlled via a controller (not shown) which
sequences the various valves discussed previously so as to
achieve the desired pressure conditions and cycles.
Most preferably, periodic agitation to the contents of
vessel 22 is effected using vibrator 60 through the entire
process. Intermittent or continuous agitation of the reactor
vessel and its contents is performed by vibrating the reactor
vessel during sterilization. Agitation enhances mass transfer
of the carbon dioxide and additives by eliminating voids in
the fluid such that the material being sterilized comes into
more complete contact with sterilant. The specific means of
agitation may be adjusted to accommodate the particular
apparatus employed and to optimize sterilization times,
temperatures, and pressure cycles. When sterilization is
complete, the vessel 22 is depressurized, the magnetic drive
66 is stopped thereby stopping the stirring impeller 64, and
the thus sterilized material removed by opening top 68 of
vessel 22.
Although the precise mechanism by which the present
invention enhances sterilization is not entirely understood at
this time it is theorized that, in conjunction with near-critical
or supercritical carbon dioxide, the chemical sterilization
additives employed in the present invention likely enhance
sterilization by increasing the acidity of the interior of the
bacterial cell, especially in the presence of water. Moreover,
additives may enhance the permeability of the cell to carbon
dioxide, irreversibly inhibit essential cellular processes, and/
or extract components required for cell viability, all of which
could possibly contribute to enhancements in sterilization
that have been observed.
The present invention will be further understood after
careful consideration is given to the following Examples.
EXAMPLE 1
The effects of using an additive in accordance with the
present invention was compared using the method described
by U.S. Pat. No. 6,149,864 to Dillow et al for inactivating B.
slearothermophilus spores. Specifically, as noted in Table 1
below, the most extreme sterilizations conditions as dis-
closed in the Dillow et al ’864 patent were employed and
resulted in only a 1 log reduction in CFUs/mL for the
experiment in which no additive was employed (Ex. A). In
contrast, a greater than 6 log reduction was achieved using
the method of the present invention (Ex. B). The additive
US 7,108,832 B2
7
was placed on a cotton ball and inserted in the chamber prior
to closure. No further additive was used.
8
remove the additive was performed by pressurizing and
de-pressurizing the reactor vessel using C02. The stirring
Agitation
Pressure # Random/ Temp Time Initial Final Log
Additive range psi cycles Directional ° C. hrs CFU/ml CFU/ml Reduction
Ex. A. Water 1500- 3 +/— 60 2 2.3 x 105 2.1 x 105 1.0
3000
Ex. B Water + 1100- 3 +/+ 60 2 2.3 x 106 0* 6.4
TFA 3000
*confirmed by turbidity test
15
EXAMPLE 2—INVENTION
The apparatus generally depicted in FIGS. 1 and 2 was
employed for this Example. A sample of B. stear0lhermo—
philus spores (1 mL) of greater than 106 CFU/mL was placed
in 16 mm diameter test tubes in a stainless steel basket.
Trifluoroacetic acid (4 mL) was transferred by syringe onto
the surface of a cotton ball placed in the basket and water (6
mL) was placed at bottom of vessel. The basket was then
loaded into the 600 mL reactor vessel. The reactor vessel
was heated to 50° C. and equilibrated with CO2 at atmo-
spheric pressure. The stirring and agitation mechanisms
were activated and the reactor vessel pressurized to 2000 psi
for 40 minutes. The CO2 pressure was then allowed to drop
to 1100 psi at a rate of 300 psi/minute. Agitation by means
of vibration of the vessel was carried out for 1 minute.
The pressurization/stirring/agitation/depres surization pro-
cess was repeated a total of three times. After the third cycle,
a series of three flushing cycles to remove the additive was
performed by pressurizing and partial de-pressurizing the
reactor vessel using C02. The stirring was stopped and the
basket was removed from the reactor vessel. The residual
CFUs were counted after serial dilution and culturing of
both treated and untreated controls.
Complete kill of bioindicators were achieved over mul-
tiple experimental evaluations. These reductions correspond
to a log reduction in CFUs of between 6.2 to 6.9.
EXAMPLE 3A—INVENTION
The apparatus generally depicted in FIGS. 1 and 2 was
employed for this Example. A sample of B. subtilis spore/
vegetative preparations (1 mL) of greater than 106 CFU/mL
was placed in a 16 mm diameter test tube in a stainless steel
basket. Acetic acid (6 mL) was transferred by syringe onto
the surface of a cotton ball placed in the basket, which was
then loaded into the 600 mL reactor vessel. The reactor
vessel was heated to 50° C. and equilibrated with CO2 at
atmospheric pressure. The stirring and agitation mechanisms
were activated and the reactor vessel pressurized to 3000 psi
for 40 minutes. The CO2 pressure was then allowed to drop
to 1500 psi at a rate of 300 psi/minute. Agitation was carried
out for 1 minute.
After depressurizing the reactor vessel, more acetic acid
(4 mL) was introduced at ambient pressure to the additive
loop via port 18 (FIG. 1). The loop was sealed and pressur-
ized to 3000 psi. The reactor vessel was the re-pressurized
through the additive loop to 3000 psi such that acetic acid
was transported into the reactor vessel.
The pressurization/stirring/agitation/depressurization/ad-
ditive addition process was repeated a total of three times.
After the third cycle, a series of three flushing cycles to
20
25
30
35
40
45
50
55
60
65
was stopped and the basket was removed from the reactor
vessel. The residual CFUs were counted after serial dilution
and culturing of both treated and untreated controls.
A log reduction in CFUs of between 6.0 to 6.9 was
observed for multiple experimental evaluations using the
procedure described above.
EXAMPLE 3B—INVENTION
Example 3A was repeated except that samples containing
less than 106 CFU/ml of B. subzilis was used. Sterilization
resulted in total kill of the B. sublilis present. It can therefore
be extrapolated from this Example that, had greater than 106
CFU/ml of B. sublilis been presented, the sterilization pro-
cedure would have resulted in a corresponding 6 log reduc-
tion in CFUs.
EXAMPLE 3C—Comparative
Example 3A was repeated except that the acetic acid was
added only once at the beginning of the procedure. Although
a 6 log reduction in CFUs was not observed, relatively high
log reductions of between 4.5 and 4.7 were observed. This
data suggests that multiple additions of acetic acid would be
needed in order to achieve the desired 6 log reduction in B.
sublilis CFUs.
EXAMPLE 3D—INVENTION
Example 3A was repeated except that pressure was main-
tained at a constant 2000 psi rather than cycling Compete
kill of bioindicators were observed over multiple tests.
These log reductions in CFUs ranged from 6.0 to 7.2.
EXAMPLE 4—INVENTION
Using the equipment and procedure in Example 1,
samples of fresh or freeze-dried bone (1 cm>106 reduction
in bacterial spores), and there was no reduction in compres-
sion strength attributes.
EXAMPLE 5—INVENTION
To evaluate the eflicacy of the improved method for
sterilization of bone tissue for implantation, human bone
tissue was saturated with a solution containing 106 CFUs/
mL of B. subtilis spores and subjected to the presented
method. The treatments were carried using the following
conditions: 4 hours, 60° C., 6 cycles form 3000—1500 psi,
constant stirring of SCD, periodic agitation of vessel, addi-
tion of 6 mL acetic acid to vessel prior to pressurization,
addition of acetic acid (4 mL) per cycle, and ending in two
5 minute flushing cycles.
The sterilized samples and unsterilized controls were
assayed for the presence of B. sublilis spores by two
methods. In the first method, bone was immersed in bacterial
media allowing germination and growth of B. sublilis
spores. Turbidity of media indicated incomplete inactivation
while clear media was complete inactivation. When cultured
for bacterial growth, none of the bone samples treated with
the above method showed detectable turbidity of the culture
medium as compared to controls (Table 2).
A sample of sterilized bone tissue was pulverized by
grinding under aseptic conditions, then cultured in media.
No turbidity was detected, indicating that the sterilization
process had permeated the bone tissue (Table 2).
TABLE 2
Sterilization of bone tissue using supercritical carbon dioxide with the
presented method
Intact Bone Pulverized Bone
Bone Inoculants Culture Culture
Treated 105 CFUs/ml No- growth No- growth
ofB. subrilis spores
Untreated 106 CFUs/ml Growth Growth
ofB. subrilis spores
EXAMPLE 6A—INVENTION
Example 3D was repeated except that peracetic acid was
employed as the sterilization additive. A log reduction in
CFUs of between 6.5 to 7.2 was observed for multiple
experimental evaluations using the procedure described
above.
EXAMPLE 6B—INVENTION
Example 6A was repeated except that pressure was main-
tained at a constant 2000 psi rather than cycling. Complete
kill of bioindicators was observed over multiple tests with
log reductions in CFUs ranging from 6.0 to 7.2.
EXAMPLE 7—Comparative
Example 3A was repeated except that the additives listed
in Table 3 below were employed under the conditions stated.
The results also appear in Table 3.
10
15
20
25
30
35
40
45
50
55
60
65
10
TABLE 3
Quantity Log
Additive Temp C. Time (vol. %) Cycles reduction
HOC1 60 3 hours 1.0 4 0—0.50
Ethanol 60—50 3 hours 1.0 4 1.24.0
Yeast Extract 60 2 hours 1.0 3 0.37—1.1
50% Citric acid 60 2 hours 1.0 3 0.03—0.62
Succinic acid 50 2 hours 1.0 3 0.25—0.29
Phosphoric acid 50 2 hours 1.0 3 0.18—0.25
Fonnic acid 50 2 hours 1.0 3 0
Malonic acid 50 2 hours 1.0 3 0—0.12
None of the additives tested in this Example showed
eflicacy to achieve at least a 6 log reduction in CFUs of B.
slearothermophilus spores.
EXAMPLE 8—Linearity of Inactivation
Example 2B was repeated except that 4.5% peracetic acid
was initially added to the vessel at 0.02 vol. % on a cotton
ball and water was added on a separate cotton ball at 1 vol.
%. B. stearothermophilus spores were inoculated onto glass
fiber filters, allowed to dry and packaged into pouches
formed of nonwoven fine polyethylene fibers (1073B
TYVEK® brand material) and served as bioindicators. Total
CFUs per filter were greater than 106. The bioindicators
were exposed to differing times of treatment with 4 repli-
cates per time point. The total remaining CFUs were then
determined and a plot was generated of log reduction in
CFUs over time (FIG. 3). Results revealed that inactivation
rates are linear and the time for a single log reduction in the
bioindicator packaged in the pouches was 14.24 minutes.
While the invention has been described in connection
with what is presently considered to be the most practical
and preferred embodiment, it is to be understood that the
invention is not to be limited to the disclosed embodiment,
but on the contrary, is intended to cover various modifica-
tions and equivalent arrangements included within the spirit
and scope of the present invention.
What is claimed is:
1. A sterilization method comprising:
(a) bringing a material in need of sterilization into contact
with a sterilant fluid comprised of carbon dioxide at
supercritical pressure and temperature conditions, and
a sterilization enhancing effective amount of between
about 0.001% to about 2.0 % based on the total volume
of a chemical sterilization additive, wherein the addi-
tive is selected from the group consisting of acetic acid,
peracetic acid, trifluoroacetic acid, acetic acid deriva-
tives or mixtures thereof, and
(b) maintaining said contact with the sterilant fluid under
said temperature and pressure conditions while
mechanically agitating for a time suflicient to achieve
a 6-log reduction or greater in colony forming units
(CFUs) of bacterial spores.
2. The sterilization method of claim 1, which comprises
agitating the sterilant fluid by stirring.
3. Apparatus for sterilizing an article in need of steriliza-
tion through the application of a sterilant fluid comprising:
a pressure vessel for containing the article in need of
sterilization
a source of supercritical carbon dioxide connected to the
pressure vessel;
a source of a liquid chemical sterilization additive
selected from the group consisting of acetic acid,
US 7,108,832 B2
11
peracetic acid, trifluoroacetic acid, acetic acid deriva-
tives or mixtures thereof connected operatively to the
pressure vessel;
means for introducing the supercritical carbon dioxide
and sterilization additive to the pressure vessel in an 5
amount of between about 0.001% to about 2.0% of the
additive based on a total volume of the sterilant fluid
introduced;
means for introducing an article in need of sterilization
into the pressure vessel so as to bring the article into
contact with the sterilant fluid at supercritical pressure
and temperature conditions and maintaining the article
in contact with the sterilant fluid under said temperature
and pressure conditions while mechanically agitating
for a time sufficient to achieve a 6-log reduction or
greater in colony forming units (CFUs) of bacterial
spores; and
a depressurization line fluid-connected to the pressure
vessel for evacuating at least some portion of the
carbon dioxide and sterilization additive from the pres-
sure vessel so as to depressurize the same.
10
15
20
12
4. Apparatus as in claim 3, further comprising a liquid-gas
separator in said depressurization line for separating carbon
dioxide gas from the liquid sterilization additive.
5. Apparatus as in claim 3, further comprising a valve in
said depressurization line to allow said at least some portion
of the carbon dioxide and sterilization additive to be evacu-
ated from the pressure vessel through the depressurization
line.
6. The sterilization method of claim 1, wherein materials
treated are selected from the group consisting of thermally
or hydrolytically sensitive, medically-important materials.
7. The sterilization method of claim 6, wherein the
materials treated are selected from the group consisting of
tissue for implantation or transplantation.
8. The sterilization method of claim 6, wherein the
materials treated are selected from the group consisting of
biodegradable and other medical polymers.
9. The sterilization method of claim 6, wherein the
materials treated are selected from the group consisting of
drugs, drug delivery systems and/or medical equipment.
* * * * *
Coments go here:
- Log in to post comments