Enhanced CO2 Absoprtion of Poly(ionic liquid)s
Enhanced CO2 Absoprtion of Poly(ionic liquid)s
Journal:
Year:
DOI:
10.1021/ma047574z
Type of document:
Language:
CO2 Absorption of Polymers of Ammonium-Based
Ionic Liquid Monomers
Jianbin Tang,1,2 Huadong Tang,1 Weilin Sun,2
Maciej Radosz1 and Youqing Shen*1
1
Department of Chemical & Petroleum Engineering, University of
Wyoming, Laramie, WY 82071; 2Department of Polymer Materials and
Engineering, Zhejiang University, Hangzhou 310027, China
as initiator. 1H NMR and element analysis indicted pure poly(ionic
liquid)s were obtain.
CH3
CH3
H2 C
H2 C
C
C O
NaBF4
C
NaBF4
C O
O
O
Introduction
Experimental
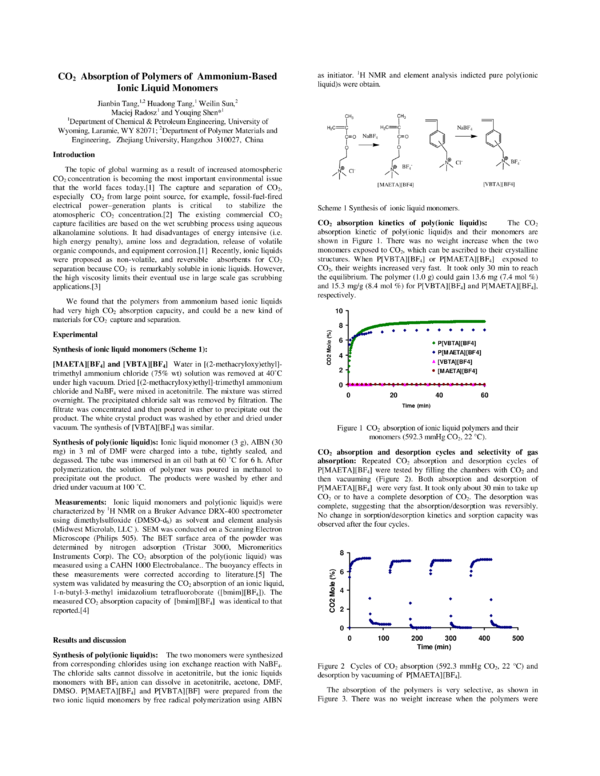
Synthesis of ionic liquid monomers (Scheme 1):
[MAETA][BF4] and [VBTA][BF4] Water in [(2-methacryloxy)ethyl]trimethyl ammonium chloride (75% wt) solution was removed at 40˚C
under high vacuum. Dried [(2-methacryloxy)ethyl]-trimethyl ammonium
chloride and NaBF4 were mixed in acetonitrile. The mixture was stirred
overnight. The precipitated chloride salt was removed by filtration. The
filtrate was concentrated and then poured in ether to precipitate out the
product. The white crystal product was washed by ether and dried under
vacuum. The synthesis of [VBTA][BF4] was similar.
Synthesis of poly(ionic liquid)s: Ionic liquid monomer (3 g), AIBN (30
mg) in 3 ml of DMF were charged into a tube, tightly sealed, and
degassed. The tube was immersed in an oil bath at 60 ˚C for 6 h. After
polymerization, the solution of polymer was poured in methanol to
precipitate out the product. The products were washed by ether and
dried under vacuum at 100 ˚C.
Measurements: Ionic liquid monomers and poly(ionic liquid)s were
characterized by 1H NMR on a Bruker Advance DRX-400 spectrometer
using dimethylsulfoxide (DMSO-d6) as solvent and element analysis
(Midwest Microlab, LLC ). SEM was conducted on a Scanning Electron
Microscope (Philips 505). The BET surface area of the powder was
determined by nitrogen adsorption (Tristar 3000, Micromeritics
Instruments Corp). The CO2 absorption of the poly(ionic liquid) was
measured using a CAHN 1000 Electrobalance.. The buoyancy effects in
these measurements were corrected according to literature.[5] The
system was validated by measuring the CO2 absorption of an ionic liquid,
1-n-butyl-3-methyl imidazolium tetrafluoroborate ([bmim][BF4]). The
measured CO2 absorption capacity of [bmim][BF4] was identical to that
reported.[4]
N
Cl-
N
BF4-
N
Cl-
BF4-
[VBTA][BF4]
[MAETA][BF4]
Scheme 1 Synthesis of ionic liquid monomers.
The CO2
CO2 absorption kinetics of poly(ionic liquid)s:
absorption kinetic of poly(ionic liquid)s and their monomers are
shown in Figure 1. There was no weight increase when the two
monomers exposed to CO2, which can be ascribed to their crystalline
structures. When P[VBTA][BF4] or P[MAETA][BF4] exposed to
CO2, their weights increased very fast. It took only 30 min to reach
the equilibrium. The polymer (1.0 g) could gain 13.6 mg (7.4 mol %)
and 15.3 mg/g (8.4 mol %) for P[VBTA][BF4] and P[MAETA][BF4],
respectively.
10
8
CO2 Mole (%)
We found that the polymers from ammonium based ionic liquids
had very high CO2 absorption capacity, and could be a new kind of
materials for CO2 capture and separation.
N
6
P[VBTA][BF4]
P[MAETA][BF4]
4
[VBTA][BF4]
2
[MAETA][BF4]
0
0
20
40
60
Time (min)
Figure 1 CO2 absorption of ionic liquid polymers and their
monomers (592.3 mmHg CO2, 22 °C).
CO2 absorption and desorption cycles and selectivity of gas
absorption: Repeated CO2 absorption and desorption cycles of
P[MAETA][BF4] were tested by filling the chambers with CO2 and
then vacuuming (Figure 2). Both absorption and desorption of
P[MAETA][BF4] were very fast. It took only about 30 min to take up
CO2 or to have a complete desorption of CO2. The desorption was
complete, suggesting that the absorption/desorption was reversibly.
No change in sorption/desorption kinetics and sorption capacity was
observed after the four cycles.
8
CO2 Mole (%)
The topic of global warming as a result of increased atomospheric
CO2 concentration is becoming the most important environmental issue
that the world faces today.[1] The capture and separation of CO2,
especially CO2 from large point source, for example, fossil-fuel-fired
electrical power–generation plants is critical
to stabilize the
atomospheric CO2 concentration.[2] The existing commercial CO2
capture facilities are based on the wet scrubbing process using aqueous
alkanolamine solutions. It had disadvantages of energy intensive (i.e.
high energy penalty), amine loss and degradation, release of volatile
organic compounds, and equipment corrosion.[1] Recently, ionic liquids
were proposed as non-volatile, and reversible absorbents for CO2
separation because CO2 is remarkably soluble in ionic liquids. However,
the high viscosity limits their eventual use in large scale gas scrubbing
applications.[3]
6
4
2
0
Results and discussion
Synthesis of poly(ionic liquid)s: The two monomers were synthesized
from corresponding chlorides using ion exchange reaction with NaBF4.
The chloride salts cannot dissolve in acetonitrile, but the ionic liquids
monomers with BF4 anion can dissolve in acetonitrile, acetone, DMF,
DMSO. P[MAETA][BF4] and P[VBTA][BF] were prepared from the
two ionic liquid monomers by free radical polymerization using AIBN
0
100
200
300
Time (min)
400
500
Figure 2 Cycles of CO2 absorption (592.3 mmHg CO2, 22 °C) and
desorption by vacuuming of P[MAETA][BF4].
The absorption of the polymers is very selective, as shown in
Figure 3. There was no weight increase when the polymers were
exposed to N2 under the same conditions, indicative of that they
selectively absorbed CO2. In N2/CO2 mixed gas, only CO2 was absorbed.
CO2 Mole (%)
8
6
CO2
4
N2
2
0
0
20
40
60
80
Time (min)
Figure 3 The selectivity of gas absorption (592.3 mmHg, 22 °C) of
P[VBIB][BF4].
Conclusions
The ammonium based poly(ionic liquid)s had high CO2 absorption
capacity: 8.5 mole % and 7.4 mole %. for P[MAETA][BF4] and
P[VBTA][BF4], respectively. The CO2 absorption and desorption are
reversibly and selectively over N2.
Acknowledgement: We thank the State of Wyoming (EORI project)
and the University of Wyoming for financial support.
References
[1] White, C. M.; Strazisar, B. R.; Granite, E. J.; Hoffman, J. S.; Pennline, H.
W. J. Air & Waste Manage. Assoc. 2003, 53: 645- 715.
[2] Herzog, H.; Drake, E.; Adams, E. CO2 Capture, Reuse and Storage
Technologies for Mitigating Global Climate Change. Report No.
DOE/DE-AF22-96PC01257, U.S. Department of Energy: Pittsburgh, PA,
1999.
[3] Eleanor D. Bates, Rebecca D. Mayton, James H. Davis, Jr. et al. J.
Am. Chem. Soc. 2002,124, 926- 927.
[4] Cesar Cadena, L. Anthony, Edward J. Maginn et al, J. Am. Chem.
Soc. 2004,126, 5300- 5308
[5] Michael D. Macedonia, Darrin D. Moore, and Edward J. Maginn,
Langmuir 2000, 16, 3823-3834
Coments go here:
- Log in to post comments