Mechanically reinforced silica aerogel nanocomposites via surface initiated atom transfer radical polymerizations
Mechanically reinforced silica aerogel nanocomposites via surface initiated atom transfer radical polymerizations
Journal:
Year:
Abstract:
Here we report the preparation of mechanically robust silica aerogel polymer nanocomposites using surface-initiated atom transfer radical polymerization. This approach was used to grow poly(methyl methacrylate) (PMMA) with low polydispersities and establish the first structure–property relationship between the grafted PMMA molecular weight and bulk physical properties of the hybrid aerogel.
DOI:
10.1039/C0JM01448F
Type of document:
Language:
View Online / Journal Homepage / Table of Contents for this issue
COMMUNICATION
www.rsc.org/materials | Journal of Materials Chemistry
Mechanically reinforced silica aerogel nanocomposites via surface initiated
atom transfer radical polymerizations†
Dylan J. Boday,a Pei Yuin Keng,b Beatrice Muriithi,a Jeffrey Pyunb and Douglas A. Loy*ab
Downloaded by University of St Andrews Library on 01 October 2012
Published on 22 July 2010 on http://pubs.rsc.org | doi:10.1039/C0JM01448F
Received 13th May 2010, Accepted 7th June 2010
DOI: 10.1039/c0jm01448f
Here we report the preparation of mechanically robust silica aerogel
polymer nanocomposites using surface-initiated atom transfer
radical polymerization. This approach was used to grow poly(methyl
methacrylate) (PMMA) with low polydispersities and establish the
first structure–property relationship between the grafted PMMA
molecular weight and bulk physical properties of the hybrid aerogel.
The addition of organic polymers to the aggregate structure of silica
aerogels has proven to be an effective strategy to enhance the
mechanical properties of these otherwise fragile materials.1 Silica
aerogels are typically formed via sol–gel processes when the solvent is
removed (generally by supercritical drying (SCD)2) without dimensional shrinkage due to drying stresses. The resultant material is
a transparent solid that is predominantly porous with air percolating
through a diaphanous network of aggregated nanoparticles.3 The
combination of low density, large pore volume, and high surface area
has made aerogels attractive for thermal4 and acoustic insulators,5
catalyst supports6 and low-k dielectric materials.7 However, their
intrinsically low density and fragility greatly complicate handling or
processing without catastrophic fracture of the material.8 Hence,
improving the mechanical properties of aerogels is an important
fundamental challenge towards achieving the full potential of these
materials for various applications.
Ideally, the reinforcement of silica aerogels should be achieved with
minimal changes to the overall density and porosity of the material as
possible. Recent efforts to prepare robust aerogels have applied
a reinforcing agent to the ‘‘necks’’ between particles in the aggregate
structure.1 Employing this general strategy, gels have been mechanically enhanced, before supercritical drying, by a number of different
approaches, such as the redistribution of existing silica in the aggregate (Ostwald ripening), the introduction of additional tetralkoxysilane monomer to the aggregate, or the growth of cross-linked
(epoxy, urethane, urea and styrene) polymer networks from the
surface of the gel.9 Recently, we reported a more rapid method for
reinforcing dried aerogels using vapor deposition of cyanoacrylates.10
In all of these cases, while the strength of the aerogels was improved,
undesirable increases in the solid state density and reduction of the
effective surface areas of the material were also observed. Based on
our observations from reinforcing aerogels with polycyanoacrylates,
we hypothesized that optimal mechanical reinforcement of aerogels
a
Department of Materials Science and Engineering, University of Arizona,
Tucson, Arizona, 85721, USA. E-mail: daloy@mse.arizona.edu; Fax: +1
520 609-6021; Tel: +1 520 621-6070
b
Department of Chemistry, University of Arizona, Tucson, Arizona, 85721,
USA; Fax: +1 520 621-8059; Tel: +1 520 609-6021
† Electronic supplementary information (ESI) available: Synthetic,
microscopic and mechanical characterization details. See DOI:
10.1039/c0jm01448f
This journal is ª The Royal Society of Chemistry 2010
could be achieved by the attachment of higher molecular weight
organic macromolecules with low grafting densities onto SiO2
surfaces without significantly increasing the density of the material.
Herein, we report the preparation of mechanically enhanced
hybrid aerogels using surface initiated atom transfer radical polymerization (SI-ATRP)11 to grow well-defined poly(methyl methacrylate)
(PMMA) onto the surface of SiO2 aerogels (Scheme 1). We observe
for the first time a tunable structure–property correlation between the
molar mass of grafted PMMA and the toughening of the hybrid
aerogel.
To ensure covalent attachment of polymers to the silica aerogel,
copolymerization of tetramethoxysilane with an a-haloester functional triethoxysilane12 was conducted and afforded a modified aerogel with an approximate surface coverage of 0.05 initiators per nm2.
The lower reactivity of the triethoxysilyl group compared to tetramethoxysilane during the ammonia-catalyzed sol–gel polymerization
ensured that the majority of initiating groups would be segregated to
the aerogel surface.13
SI-ATRP of MMA was conducted in solution using a copper(I)bromide, copper(II)bromide, and 4,40 -dinonyl-2,2-bipyridine (dNbpy)
catalyst system in the presence of a monolithic gel. Care was taken to
completely exchange the solvent in the gels with the ATRP monomer
and catalyst at room temperature before heating to start the polymerizations. A target degree of polymerization of [M]o/[I]o ¼ 200 was
chosen. Polymerization times were varied from 6–40 h, thus allowing
for control over the grafted polymers molecular weight (Table 1). The
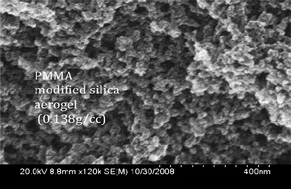
difference in morphology between a polymer free aerogel and an
aerogel composite after SI-ATRP is pronounced and appears to be
thickened (Fig. 1).
Scheme 1 Formation of initiator-modified gel, ATRP growth of
PMMA on surface and supercritical drying to afford a silica–PMMA
composite aerogel.
J. Mater. Chem., 2010, 20, 6863–6865 | 6863
View Online
Table 1 Properties of silica, PMMA–silica aerogel composites and polymer characterization
Aerogel
polymerization
time/h
PMMA
Mw/g molÀ1
PDI
Aerogel
density/g cmÀ3b
Flexural
strength/kPa
Surface
area/m2 gÀ1
Mean
pore dia./nm
Pore
volume/cm3 gÀ1
0a
6
20
30
40
—
20 390
43 013
63 284
75 246
—
1.55
1.22
1.68
1.82
0.092 Æ
0.116 Æ
0.162 Æ
0.236 Æ
0.330 Æ
3.4 Æ 0.1
15.4 Æ 0.49
30.2 Æ 0.52
48.5 Æ 1.3
63.5 Æ 1.7
1483
403
301
221
185
11.4
23.6
32.8
27.0
26.4
9.76
5.83
3.47
0.96
0.81
Downloaded by University of St Andrews Library on 01 October 2012
Published on 22 July 2010 on http://pubs.rsc.org | doi:10.1039/C0JM01448F
a
0.001
0.017
0.032
0.038
0.072
Silica aerogel prepared without PMMA. b Density values are an average from four gels.
Determination of tethered PMMA molar mass was achieved by
dissolution of SiO2 gels with hydrofluoric acid (HF) and size exclusion chromatography (SEC) of recovered polymers. Controlled SIATRP was achieved, as evidenced by SEC of cleaved PMMA indicated that polymers in the range of Mn ¼ 13 to 43 kg molÀ1 were
grown with polydispersities in the range of Mw/Mn ¼ 1.2 to 1.8.
After SCD, aerogel composites were slightly blue tinted due to
residual copper catalyst that was as high as 1% of the original copper
concentration in aerogels that were treated for 40 hours.
The initiator efficiencies ranged from 21–33%. While these
molecular weight distributions were more broad than those observed
for typical homogeneous MMA ATRP in solution, these higher
polydispersities are typical for SI-ATRP systems observed from SiO2,
concave surfaces and polysilsequioxane colloidal initiators.11a,14
Additionally, it is anticipated that the complex environment of aerogel surfaces contained sterically inaccessible initiation sites that also
affected the overall control of the SI-ATRP.
Fig. 1 (A) SEM image of silica aerogel with pendent a-haloester functional silane and (B) silica aerogel–PMMA composite after 30 h polymerization time.
6864 | J. Mater. Chem., 2010, 20, 6863–6865
Thermogravimetric analysis of poly(methyl methacrylate) grafted
silica aerogels (PMMA-g-SiO2) also confirmed that the organic
content of hybrid nanocomposites scaled with increasing molar mass
of SI-ATRP grown PMMA (Fig. 2).
By comparing the analyses of samples taken near the center of the
monolithic aerogels to those from the surface, we were able to show
that with excess monomer present early in the ATRP treatment the
concentration of PMMA is relatively uniform with the difference in
weight-percent organic between the center and exterior of the aerogels differing only by a couple of percent. After twenty hours of
ATRP, the difference in organic content between the interior and
exterior of the aerogels has increased to 10%. We believe that this is
due to monomer depletion within the gel as the polymerization
proceeds. As polymer forms, diffusion of more monomer into the gel
is restricted leading to larger polymer concentrations and higher
molecular weights on the exterior of the gel compared to the interior
of the gel and increasing PMMA polydispersity in the aerogel.
Similar, surface heterogeneities were observed in the CVD polymerization of cyanoacrylates on silica aerogels modified with amine
groups15 and may be analogous to monomer diffusion/polymerization approaches to gradient refractive index materials.
The effects of grafting PMMA to strengthen the aerogels are
shown in Fig. 3. Triplicate (or more) aerogels were analyzed at each
represented point using a three-point flexural bend analysis according
to ASTM C1684. Flexural strengths of pure silica aerogels as
a function of density are provided as a baseline (triangles in Fig. 3).
The diamonds represent the strengths of the aerogels with grafted
PMMA. The first point (r ¼ 0.092 g cmÀ3) is that of the initiator
modified aerogels with no PMMA. These aerogels were slightly
weaker (340 Æ 10 kPa) than a pure silica aerogel. The minor
Fig. 2 TGA analyses of samples from the interior and exterior of silica
aerogels ATRP modified with PMMA for 6 (bold) and 20 hours (dashed).
This journal is ª The Royal Society of Chemistry 2010
View Online
Downloaded by University of St Andrews Library on 01 October 2012
Published on 22 July 2010 on http://pubs.rsc.org | doi:10.1039/C0JM01448F
due to the filling of the micropores (
Coments go here:
- Log in to post comments