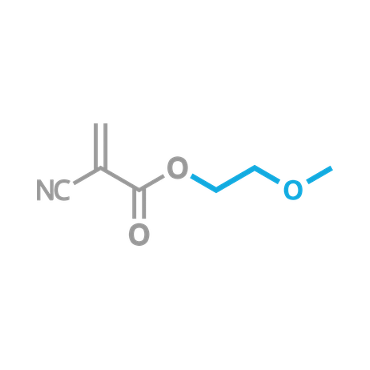
This, or the closely related 2-Ethoxyethyl 2-Cyanoacrylate, is the most commonly known odorless, non-blooming liquid CA
English
product
β-Methoxyethyl Cyanoacrylate
categories
Monomers
Hide in products list
1