Synthesis of Functionally Substituted Cyanoacetates
Synthesis of Functionally Substituted Cyanoacetates
Folder:
Journal:
Year:
Abstract:
Some new cyanoacetates were synthesized and characterized. They are precursors for α-cyanoacrylates used as rapidly polymerized, cold-hardening adhesives.
DOI:
10.1007/BF00698435
Type of document:
Language:
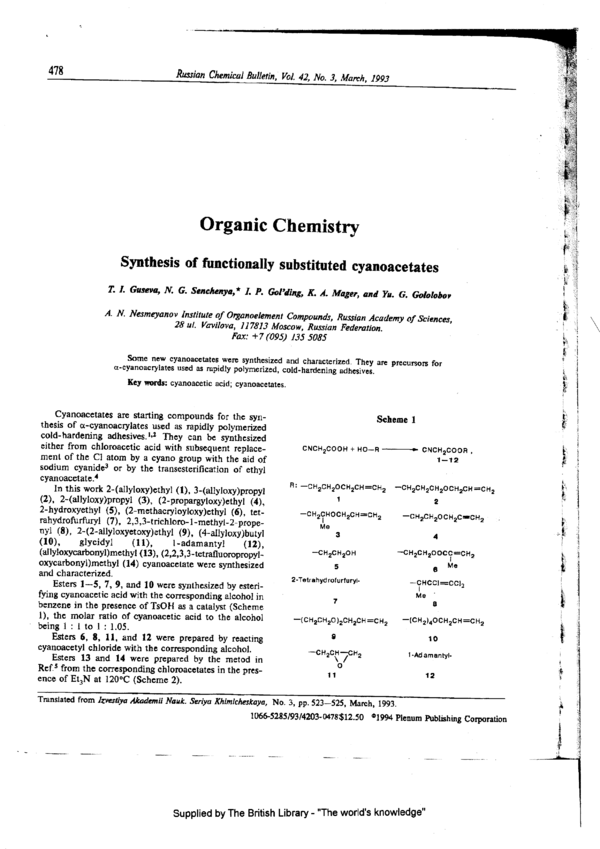
In this work 2-(ailyloxy)ethyl (1), 3-(allyloxy)propyl R: ’°”2°”=°°"*°“=°"'2 "°”"°”2°”2°°”’°”=°”2
(2). 2-(allyloxwpropyl (3). (2—proparsyloxy)ethy1 (4). ' 2 ;
2-hydroxyethyl (5), (2—methacryloyloxy)ethyl (6), tet- —CH2CHOCH2CH=CH2 —CH2CH20CH2cscH2 M
rahydrofurfuryl (7), 2,3,3-trichloro-1-methyl—2-prope— ([19 p
try} (8), 2—(2-ally1oxyetoxy)ethyl (9), (4-allyloxy)b'utyl 3 4
(10), giycidyi (11), 1-adamantyl (12), _ _ _ .
(allyloxycarbonynmethyl (13), (2,2,3,3-tetrafluoropropyl- _CH2°H2°H CH2cH2O°ccl:-CH2 3
oxycarbonyl)methyl (14) cyanoacetate were synthesized 5 3 Me
and Ch3-7actCriZ°d- 2-Tetrahydroturturyh _ci-icc1=cc12 j V3
Esters l—~5, 7, 9, and 10 were synthesized by esteri- I _ i.
fying cyanoacetic acid with the corresponding alcohol in 7 M° a
benzene in the presence of TsOH as a catalyst (Scheme 1
1). the molar ratio of cyanoacetic acid to the alcohol —(CH2CH20)2CH2CH=CH2 -(CH2)40CH2CH=CH-2 ,‘ §
- being 1 : 1 to 1 : 1.05. §
Esters 6, 8, 11, and 12 were prepared by reacting 9 ‘° ‘
cyanoacetyl chloride with the corresponding alcohol. _.cH2cH—cH2 1_Ad ama,,,y,_ =
Esters 13 and 14 were prepared by the metod in \o/ 1
Ref.5 from the corresponding chloroacetates in the pres- 1 1 12
ence of Et3N at 120°C (Scheme 2). -V
478 Russian Chemical Bulletin, Vol. 42, No. 3, March, 1993
Organic Chemistry
Synthesis of functionally substituted cyanoacetates
T. I. Guseva, N. G. SencIrenya,* I. P. Gol’ding, K. A. Mager, and Yu. G. Gololobov
A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences,
.28 ul. Vavilova, 117813 Moscow, Russian Federation.
Fax: +7 (095) 135 5085
*""vr~r~ézw ‘ « ‘ r
_ ii
Some new cyanoacetates were synthesized and characterized. They are precursors for 1_
oucyanoacryiates used as rapidly polymerized, cold-hardening adhesives.
Key words: cyanoacetic acid; cyanoacetates.
4
Cyanoacetates are starting compounds for the syn- Scheme 1
thesis of on-cyanoacryiates used as rapidly polymerized "
coid—hardening adhesives.‘-1 They can be synthesized
either from chloroacetic acid with subsequent replace- CN°’'‘2C°°“ ‘“ "0"" “’—" °N°”2°°°9 -
ment of the Cl atom by a cyano group with the aid of ‘*1’
sodium cyanide3 or by the transesterification of ethyl
cyanoacetate.‘
W-*"*'
_W, -,,,__,.
Translated from Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 3, pp. 523-525, March, 1993.
1066-5285/93/420?» 0478'$12.50 °1994 Plenum Publishing Corporation
r..ir‘,'_,,,
Supplied by The British Library - "The world's knowledge"
Synthesis of functionally substituted cyanoacetates Russ.Chem.BuI1'., Vol. 42, Na. 3, March, 1993 479
Table 1. Yields, physical properties, and ‘H NMR spectral data of the cytmoacetates CNCI-l2CO0R
Compound Yield (96) Bp ['C (T 011)] 7191” 11430 ‘H NMR (solvent, 5)
1 so 127(1) 1.4495 1.0894 cc1,,; 3.57 (s, 2 H, cH,cN); 3.70 (m, 2 H, CHIOR);
4.00 (m, 2 H, cH,cH=); 4.33 (m, 2 H, COOCH2)
2 81 11o(0.1) 1.449 1.063 (cD,),co; 1.90 (m, 2 H, cH,cH,cH,);
3.51 (1, 2 H, cH,oR); 3.70 (s, 2 H, cH,cN);
4.00 (m, 2 H, c1;1,cH=); 4.21 (1, 2 H, coocH,)
3 72 126(4) 1.446 1.050 (cD,),co; 1.26 (11, 3 H, CH3); 3.51 (1, 2 H, ocH,cH);
3.75 (1, 2 H, Cl-IZCN); 3.94 (m, 2 H, cH,cH=);
5.06 (m, 1 H, cH,cH)
4 51 135(1) 1.4600 1.1450 cc1,,; 2.40 (1, 1 H. =CH); 3.40 (s. 2 H, cH,cN);
3.80 (m, 2 H, cH,o); 4.16 (11, 2 H, cH,ca);
4.33 (m, 2 H, coocH,)
5 45 139(2) 1.4500 1.2500 (CD3)2CO; 3.52(s, 2 H, CH2CN); 4.02(m, 2 H, C1120}-I);
4.40 (s, 1 H, OH); 4.55 (m, 2 H. COOCH2:’
6 35 133(1) 1.4626 1.1426 CC14; 1.90 (s, 3 H, CH3); 3.40 (s, 2 H, CH2CN);
4.26 (m, 2 H, C1-1,0); 4.44 (In, 2 H, COOCH2);
5.63, 6.21 (d, 2 H, CH2=)
7 82 121(1.5) 1.4583 1.1559 CDCI3 ; 1.30 (In, 4 H, Cfl2CH2CH);
1.66 (m, 2 H, CH;CH20); 1.88 (S, 2 H, CH2CN);
3.5 (m, 2 H, OCHZCH); 3.84 (m, 1 H, CH)
8 65 125(0.5) 1.4970 1.4132 CCI4; 1.33 (d, 3 H, CH3); 3.32 (s, 2 H, CH2CN);
4.73 (q, 1 H, CH)
9 65 160(2) 1.4575 1.1009 CCI4; 3.53 (s, 2 H, CH3CN); 3.63 (m, 4 H, CH2OCi-12);
3.75 (m, 2 1-1, C1-12OCH2CH=);
4.00 (m, 2 H, CH,2CH=CI-12); 4.31 (m, 2 H, COOCH2)
10 69 130(1) 1.4530 1.037 (CD3)2CO; 1.71 (m, 4 H, CH1C};l2CH,Cl-12);
3.72 (S, 2 H, CHQCN); 3.40 (m, 2 H, CI:I2OCH2CH=);
3.91 (m, 2 H, Cfl2Cl-I=); 4.30 (m, 2 H, COOCH2)
11 40 30(4) 1.4157 1.0965 cc1,; 2.5-3.5 (m, 5 H, glycidyl fragment);
3.60 (s, 2 H, cH,cN)
12 20 Mp 94 — — cc1,; 1.61, 2.03, 2.16 (m, 6 H, 6 H, 3 H, Ad-fragment);
3.60 (s, 2 H, cH,cN)
13 so 125(1.5) 1.44 1.1100 (c13,,,co; 3.73 (s, 2 H, cH,cN);
4.66 (m, 2 H. ocH,cH=); 4.73 (s, 2 H, coocH,c(o))
14 34 132(1.5) 1.4030 1.4517 (cD,),co; 3.80 (s, 2 H, cH,cN);
4.70 (t, 3J19F_1H = 13.4 Hz, 2 H, CHQCF2);
4.90 (5, 2 H, 0CH2C(O));
6.29 (tt, 2J19F.—1H = 47.8 Hz, 3J19F_1H = 4.5 Hz, 1 H,
CF21-I)
Note. For compounds 1, 2, 3, 9, 10, 13 ‘H NMR (6) : 5.1 ‘1-5.35 (m, 2 H, CH2=), 5.90 (m, 1 H, CH=).
Scheme 2 Esters ll, 13, and 14 are liquids, while 12 is a solid.
All the esters obtained we stable during storage. Their
structures are supported by IR and ‘H NMR spectra,
ClCH,0OOR + CNCHZCOOK -—. cNcH2cO0CH,COOR and the yields and properties are presented in Table 1.
13 1 4 The IR spectra of the compounds synthesized contain
13; Q = ..cH2cH=cH2 ' absorbtion bands that are characteristic of all cyanoacc—
tates at 1720—1760 (C=0) and 2250-2260 (C=N) cm“,
um: —cH’CF’cF’H as well as absorption bands at 1620-1640 cm"
Supplied by The British Library - "The world's knowledge"
~..-3.
480 Russ. Chem. BulI., Vol. 42, N0. 3, March, 1993 Gusgva er a[_
3 I A] 11 All
C(l) 71 60 72 4 -0 ll 78 9 -7 3
Ca) 72 36 71 9 0 5 65 9 6 0
(C=C) for 1-4, 6, 8-10, 13 which contain an alkenyl
radical in the esteric group, at 2140 and 3300 cm“
(C:C) for 4, at 800 cm“ (C-C1) for 8, and at 860 and
1240 cm“ for epoxide 11.
The unsaturated glycol monoethers used for the syn-
theses of 1-4, 9, and 10 were obtained by Williamson
reactions-" of glycol alkoxides with allyl or propargyl
halides. “C NMR was used to establish whether com-
pound 3 forms with structure I or 11. The calculated‘
chemical shifis of C(l), C(2), and C(3) for structures I
and 11 are presented in Table 2 and compared with the
empirical data for compound 3. It is seen from the
results presented that the empirical chemical shifts of
CH), C(2) and C(3) are in good agreement with the
calculated values for structure 1. Thus, it has been
established that compound 3 has structure I.
1 2
CNCH2COOCH2(;JHOCH2CH‘—-‘CH2
I 3CH3
2 1
cNcH2cooc';HcH2ocH,,cH=cH2
3CH3
11
Experimental
The ‘H and “C NMR spectra of 10-20 913 solutions in
CC], or deuterated solvents were measured on a Bruker
WP-200-SY spectrometer (200.13 and 50.32 MHz, respective-
ly) with hexamethyldisiloxane (6 = 0.05) as an lrrtemal stand-
ard. The IR spectra were recorded on a UR-20 spectrometer in
KBr pellets.
The chemically pure starting compounds were purified by
vacuum distillation or crystallization.
The purity of the cyanoacetates was determined by GLC on
a Chrorn 5D chromatograph equipped with a katharometer and
a stainless steel column packed with 10 96 XE-60 on Chrornaton
N-AW. Helium was used as a carrier gas (0.5X10“ m3 5").
The samples were injected as 10% solutions in CH2Cl1.
2-(A1lyloxy)propyl cyauoacetate (3). A mixture of 42.5 g
(0.5 mol) of cyanoacetic acid, 61 g (0.55 mol) of
2-(al1yloxy)propanol, and 2 3 of TSOH in 150 mi, of dry
benzene was heated under reflux in a round-bottom flask
equipped with a Dean-Stark trap. After the calculated volume
of water (9 mL) was distilled off, the solvent was evaporated
in vacua. Fractionation of the residue by vacuum distillation
under He gave 66 g (72%) of 3. Found (96): C, 58.85; H, 7.12;
N, 7.85. C91-IIZNO3. Calculated (%): C, 59.02; H, 7.10;
N, 7.65. IR, v(cm“): 1720 (C=O), 1640 (C=C), 2250 (CsN).
Compounds 1, 2, 4, 5, 7, 9, and 10 were synthesized in a
similar manner.
1-Adaantyl cyanoncetete (12). To :1 solution of 42.5 g
(0.5 mol) of cyanoacetic acid in 250 mL of ether was added
104.2 g of P05 with stirring and cooling to keep the tempera-
ture at 20°C. After the evolution of HCl ceased, the solvent
and POCI3 were distilled off in vacuo. Vacuum distillation of
the residue gave 45.5 g (89%) of cyanoacetyl chloride, bp 57-
58°C (0.1 Torr).
To a solution of 75 g (0.44 mol) of 1-adamantanol in
250 mL of benzene eyanoucctyl chloride (45.5 g, 0.38 mol)
was added dropwise at --20°C, and the mixture was heated at
55°C until the evolution of HCl ceased. After cooling, the
reaction mixture was passed through silica gel LS 40/100 (the
eluent was benzene), the solvent was evaporated, and recrys-
tallzation of the residue from ethanol gave 193 g (20%) of 12,
mp 94°C. Found (96): C, 71.30; H, 7.70; N, 6.32. CUHWNO1.
Calculated (96): C, 71.23; H, 7.76; N, 6.39. IR, v(cm“): 1720
(C=C), 2250 (Cam, 2860, 2920 (CH, CH2, Ad fragment).
Compounds 6 and 8 were synthesized in a similar man-
ner.
(2,2,3,3-Tetralluoropropy1oxycarbonyl)methyl cyanoacetate
(14). A mixture of 151.05 g (1.14 mol) of 2,2,3,3-tetrai1uoro-
propyl chloroacetate, 40 g (0.33 mol) of potassium cyanoace-
tate, and 1.2 mL of triethylamine was heated at 120°C for 1 h.
The precipitate formed was filtered off and washed with ether.
The combined filtrates were evaporated in vacuo and 53.5 g (84
96) of product 14 was obtained. Found (96): C, 36.97; H, 2.61;
F, 29.84; N, 5.10. C8H7F,NO4. Calculated (95): C, 37.35; H,
2.72; F, 29.57; N 5.45. IR, v (cm"): 1720 (C=C), 2260
(CEN), 1070-1250 (CF2).
Ester 13 was obtained in a similar manner.
References
l.V. V. Korshak, A. M. Polyakova, K. A. Mager, and V. N.
Semyantsev, USSR lnventor’s Certificate No. 696,013
Byull. Izobr-et., 1979, No. 41, 96 (in Russian).
2. Yu. G. Gololobov, K. A. Mager, '1‘. I. Guseva. N. G.
Senchenya, I. V. Lopatina, and N. V. Klimentova, Absrr. of
1111- Union Cor;/f «Radical Polymerization», Gor‘kii, 1989, 68
(in Russian). ‘
3. Preparative Organic Chemistry, Ed. trans1., Gos. Nauchno-
Tekhn. Izd. Khim. Literatury, Moscow, 1959, 437 (in Rus-
sian).
4. A. M. Polyakova, K. A. Mager, and E. A. Borisova, Zh. Org.
Khim., 1967, 3, 1205 (J. Org. Chem. USSR, 1967, 3, No. 7
(Engl. Transl.)].
5. V. S. Etlis, L. M. Degtyareva, and N. N. Trofimov, Zr.
Prikl. Khim., 1971, 44, 937 [J. Appl. Chem. USSR, 1971, 44,
No. 4 (Engl. Transl.)].
6. B. I. lonin, B. A. Ershov, and A. 1. 1(o1’tsov, NMR Spectros-
copy in Organic Chemistry, Khimyia, Leningrad, 1983, 146
(in Russian).
Received February 12, 1992
Supplied by The British Library - "The world's know|edge"
Coments go here:
- Log in to post comments