t-Butyl Cyanoacetate
t-Butyl Cyanoacetate
Folder:
Journal:
Year:
Type of document:
Language:
Organic Syntheses, Coll. Vol. 5, p.171 (1973); Vol. 41, p.5 (1961).
t-BUTYL CYANOACETATE
[Cyanoacetic acid, tert-butyl ester]
Submitted by Robert E. Ireland and Michael Chaykovsky1.
Checked by Max Tishler and Arthur J. Zambito.
1. Procedure
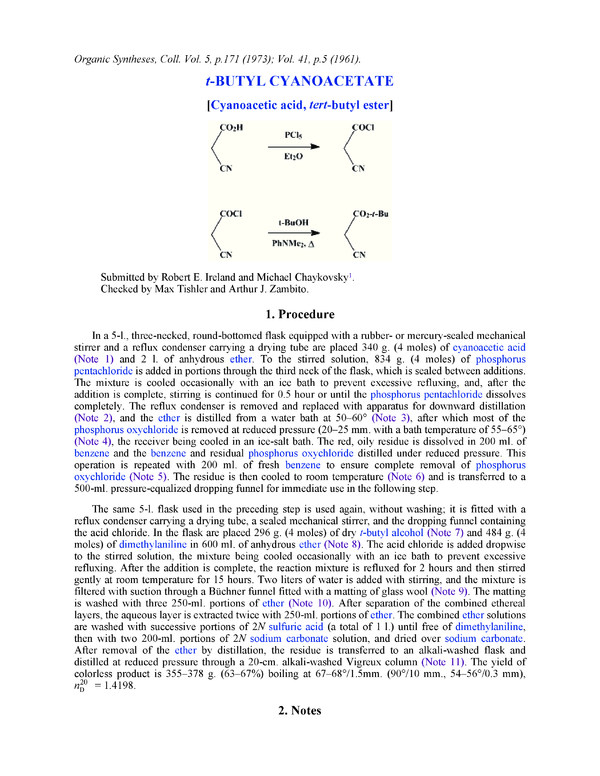
In a 5-l., three-necked, round-bottomed flask equipped with a rubber- or mercury-sealed mechanical
stirrer and a reflux condenser carrying a drying tube are placed 340 g. (4 moles) of cyanoacetic acid
(Note 1) and 2 l. of anhydrous ether. To the stirred solution, 834 g. (4 moles) of phosphorus
pentachloride is added in portions through the third neck of the flask, which is sealed between additions.
The mixture is cooled occasionally with an ice bath to prevent excessive refluxing, and, after the
addition is complete, stirring is continued for 0.5 hour or until the phosphorus pentachloride dissolves
completely. The reflux condenser is removed and replaced with apparatus for downward distillation
(Note 2), and the ether is distilled from a water bath at 50–60° (Note 3), after which most of the
phosphorus oxychloride is removed at reduced pressure (20–25 mm. with a bath temperature of 55–65°)
(Note 4), the receiver being cooled in an ice-salt bath. The red, oily residue is dissolved in 200 ml. of
benzene and the benzene and residual phosphorus oxychloride distilled under reduced pressure. This
operation is repeated with 200 ml. of fresh benzene to ensure complete removal of phosphorus
oxychloride (Note 5). The residue is then cooled to room temperature (Note 6) and is transferred to a
500-ml. pressure-equalized dropping funnel for immediate use in the following step.
The same 5-l. flask used in the preceding step is used again, without washing; it is fitted with a
reflux condenser carrying a drying tube, a sealed mechanical stirrer, and the dropping funnel containing
the acid chloride. In the flask are placed 296 g. (4 moles) of dry t-butyl alcohol (Note 7) and 484 g. (4
moles) of dimethylaniline in 600 ml. of anhydrous ether (Note 8). The acid chloride is added dropwise
to the stirred solution, the mixture being cooled occasionally with an ice bath to prevent excessive
refluxing. After the addition is complete, the reaction mixture is refluxed for 2 hours and then stirred
gently at room temperature for 15 hours. Two liters of water is added with stirring, and the mixture is
filtered with suction through a Büchner funnel fitted with a matting of glass wool (Note 9). The matting
is washed with three 250-ml. portions of ether (Note 10). After separation of the combined ethereal
layers, the aqueous layer is extracted twice with 250-ml. portions of ether. The combined ether solutions
are washed with successive portions of 2N sulfuric acid (a total of 1 l.) until free of dimethylaniline,
then with two 200-ml. portions of 2N sodium carbonate solution, and dried over sodium carbonate.
After removal of the ether by distillation, the residue is transferred to an alkali-washed flask and
distilled at reduced pressure through a 20-cm. alkali-washed Vigreux column (Note 11). The yield of
colorless product is 355–378 g. (63–67%) boiling at 67–68°/1.5mm. (90°/10 mm., 54–56°/0.3 mm),
20
nD = 1.4198.
2. Notes
1. Cyanoacetic acid of 98% purity, obtained from Kay-Fries Chemicals, Inc., was used.
2. A Claisen head with a condenser leading into a flask with a suction arm connected to a drying tube is
suitable. Ground-glass joints are recommended.
3. A large bucket containing water and placed on a steam bath serves as a suitable water bath. The
temperature is easily controlled between the limits mentioned.
4. The ether is removed from the receiving flask before the phosphorus oxychloride is distilled. A
drying tube should be placed between the suction arm of the flask and the water pump, which serves as
the source of suction. The reaction mixture may be stirred during the distillation of the oxychloride, or
the stirrer may be removed and replaced with a capillary ebulliator tube to which is attached a ballon
filled with dry nitrogen.
5. The checkers found that the distillation with benzene ensures a more complete removal of phosphorus
oxychloride which, if still present, interferes in the subsequent step and a lower yield of product results.
6. The submitters found that on several occasions, when the residue was not cooled before transfer, it
began to generate considerable heat while standing in the dropping funnel and resulted in the total
carbonization of the acid chloride.
7. The submitters dried the t-butyl alcohol by refluxing it over calcium hydride overnight and
distillation in a moisture-free apparatus. The checkers found that stirring the t-butyl alcohol at 60–70°
over calcium hydride for several hours and then distilling the alcohol, using an air condenser, is a
satisfactory procedure. When the t-butyl alcohol is refluxed, the alcohol vapors condense and solidify
(m.p. 24–25°) in the reflux condenser and cause clogging.
8. These reagents should be weighed out beforehand in order to prevent delay in commencing with this
step.
9. The filtration removes some tarry resinous material which would otherwise interfere in the separation
of the layers. The checkers found that unless the filtrate is recycled through the same matting several
times, to remove practically all the tarry residue, the separation of layers and the subsequent extractions
prove troublesome owing mainly to emulsion formation.
10. The checkers found that a considerable amount of product is withheld by the residue on the glasswool matting. The product is extracted by placing the matting in a beaker, stirring with ether, and
filtering. This procedure is repeated twice, and the ether extracts are combined with the original filtrate.
11. The distilling flask and Vigreux column to be used should be washed with 25% aqueous sodium
hydroxide solution, rinsed three times with water, and then dried. Alternatively, about 1 g. of anhydrous
potassium carbonate may be added to the residue before distillation.
3. Discussion
t-Butyl cyanoacetate has been prepared from t-butyl bromoacetate and potassium cyanide in
methanol2 and from t-butyl chloroacetate and potassium cyanide in methyl Cellosolve.3 The present
method is an adaptation of that of Beech and Pigott4 and is similar to the Organic Syntheses preparation
of t-butyl acetate.5
4. Merits of Preparation
The present preparation employs a method of considerable scope which gives much better yields
and is considerably less laborious than other methods for the preparation of t-butyl cyanoacetate. The
compound is of specific interest since, for example, it may be used in any reaction where ethyl
cyanoacetate is used (condensation reactions, etc.), but it has the added advantage that the carbo-tbutoxy group, which may serve in conjunction with the α-cyano group to activate the α-hydrogens (for
cyanoethylations, etc.), may be later removed simply by pyrolysis of the compound.6
References and Notes
1.
2.
3.
4.
5.
University of Michigan, Ann Arbor, Mich.
B. Abramovich and C. R. Hauser, J. Am. Chem. Soc., 64, 2271 (1942).
Private communication, W. S. Johnson, University of Wisconsin.
W. F. Beech and H. A. Pigott, J. Chem. Soc., 423, (1955).
C. R. Hauser, B. E. Hudson, B. Abramovitch, and J. C. Shivers, Org. Syntheses, Coll. Vol. 3, 142
(1955).
6. S.-O. Lawesson, E. H. Larsen, and H. J. Jakobsen, Arkiv Kemi, 23, 453 (1965).
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
potassium carbonate (584-08-7)
sulfuric acid (7664-93-9)
Benzene (71-43-2)
methanol (67-56-1)
ether (60-29-7)
sodium hydroxide (1310-73-2)
phosphorus pentachloride (10026-13-8)
sodium carbonate (497-19-8)
nitrogen (7727-37-9)
potassium cyanide (151-50-8)
Phosphorus Oxychloride (21295-50-1)
Ethyl cyanoacetate (105-56-6)
cyanoacetic acid (372-09-8)
dimethylaniline (121-69-7)
methyl Cellosolve (109-86-4)
t-butyl alcohol (75-65-0)
calcium hydride (7789-78-8)
t-BUTYL CYANOACETATE,
Cyanoacetic acid, tert-butyl ester (1116-98-9)
t-butyl acetate (540-88-5)
t-butyl chloroacetate (107-59-5)
t-butyl bromoacetate (5292-43-3)
Copyright © 1921-2005, Organic Syntheses, Inc. All Rights Reserved
Coments go here:
- Log in to post comments