Improved Hot Strength Cyanoacrylate Adhesive Composition
Folder:
Year:
Abstract:
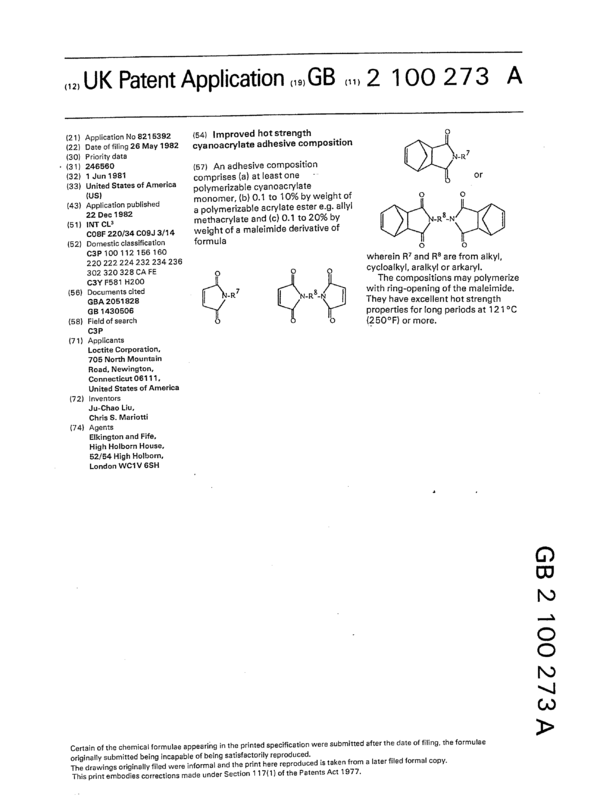
An adhesive composition comprises (a) at least one polymerizable cyanoacrylate monomer, (b) 0.1 to 10% by weight of a polymerizable acrylate ester e.g. allyl methacrylate and (c) 0.1 to 20% by weight of a maleimide derivative of formula wherein R<7> and R<8> are from alkyl, cycloalkyl, aralkyl or arkaryl. The compositions may polymerize with ring-opening of the maleimide. They have excellent hot strength properties for long periods at 121 DEG C (250 DEG F) or more.

Type of document:
Language:
(12)UK Patent Application (19)GB (11) 2 100 273 A
(21) Application No 3215392 (54) Improved hot strength o
(22) Date of filing 26 Mav1932 cyanoacrylate adhesive composition
(30) Priority data
’ (31) 245560 (57) An adhesive composition
(32) 1 "_“" 1981 _ comprises (a) at least one -* or
(33) :JL?:)ed States °f Amen“ polymerizable cyanoacrylate _
monomer, (b) 0.1 to 10% by weight of
. . . 0 0
(43) §§°,','::"1°§3‘;”b"s"ed a polymerizable acrylate ester e.g. allyl
(51) WT c._a methacrylate and (c) 0.1 to 20% by _R8_N
co3|= 22o/34 C09,] 3/14 weight of a maleimide derivative of
(52) Domestic classification formula 0 O
C3P10011215616O
220 222 224 232 234 235 wherein R7 and R8 are from alkyl,
302 320 328 CA FE O 0 0 cycloalkyl, aralkyl or arkaryl.
C3Y F581 H200 The compositions may polymerize
(56) Documents cited _ 7 with ri,-,g_0penin of the I - -d
GBA 2051323 -R N’R8’N 1 They have excellgnt hot sTr:rie;;1' e.
(58) gzlgifgiojh properties for long periods at 121°C
0 (250°F) or more
C3P —- '
(71) Applicants
Loctite Corporation,
705 North Mountain
Road, Newington,
Connecticut 06111 ,
United States of America
(72) Inventors
Ju-Chao Liu,
Chris 8. Mariotti
(74) Agents
Elkington and Fife.
High Holborn House.
52/54 High Holborn.
London WC1V 6SH
Certain of the chemical formulae appearing in the printed specification were submitted after the date of filing, the formulae
originally submitted being incapable of being satisfactorily reproduced.
The drawings originally filed were informal and the print here reproduced is taken from a later filed formal copy.
This print embodies corrections made under Section 1 17(1) of the Patents Act 1977.
V€[_Z OOL Z89
2199273
1I2
COMPOSITION ‘G’ CHART OF THERMOGRAM
Iliiilllllllllll
IIIIIHIIIIIIIIII
IIIIIIIIIIIIIIII
lllllllfillllllll
IIIIIIIIINIIIIII
IIIIIIIII|lIIII|
IIIIIIIIIINIIIII
!!IIIIIIIIIIIII|
200 300 400
TEMPERATURE °c '
F7ug.1
°/. WEIGHT LOSS
8 8
70
80
90
100
WEIGHT LOSS
2/2
COMPOSITION ‘A’
CHART
OF THERMOGRAM
TEMPERATURE
°C
2100273
GB 2 100 273 A 1
.
10
15
20
25
30
35
40
45
SPECIFICATION
Improved hot strength cyanoacrylate adhesive composition
This invention relates to certain liquid adhesive and sealant compositions which, upon cure, have
improved structural strength properties at elevated temperatures and improved resistance to thermal
degradation. 5
Adhesive and sealant compositions based on cyanoacrylate monomers are known in the art.
Typical examples of patents relating to cyanoacrylate adhesives are U.S. Patents 2,784,215 to Joyner,
2,794,788 to Coover et al., and British Patent 1,196,069 to O’Su||ivan. Cyanoacrylate adhesive
compositions are extremely sensitive, and great care must be exercised in their formulation. Cure
(polymerization) is generally considered to be initiated by an anionic mechanism, with water being a
sufficiently strong base to initiate the cure under most circumstances. The adhesives remain shelf—stable
items of commerce as long as they are suitably packaged, but when placed on a substrate to be bonded
and exposed to atmospheric and surface moisture, cure generally is instituted in a relatively short period
of time, generally less than one (1) minute and on many surfaces, within a matter of seconds. This
exceptional cure speed offers numerous advantages, particularly to those who use adhesive bonding in
production line applications. However, a major shortcoming which has heretofore limited the areas of
applicability of cyanoacrylate adhesives has been the relatively low thermal resistance of the cured
bonds. Bonded assemblies frequently are exposed to continuous operating temperatures substantially
above normal room temperature, and adhesive assemblies must retain reasonable strength for
substantial periods of time at these elevated temperatures to retain their usefulness.
In addition to strength retention by the adhesive at elevated temperatures (i.e., hot strength), the
adhesive bonds must not be unduly affected by continuous or repeated exposure to elevated
temperatures (resistance to heat degradation). In the prior art it has not been possible to prepare a
cyanoacrylate adhesive composition which produced substantial cross-linking on cure, even when
ostensively di—functiona| cyanoacrylate monomers were used. Further, because of the extreme reactivity 25
of the cyanoacrylate monomer, there have been substantial limitations upon addition of other
ingredients, such as cross—linking agents or co—monomers, in order to improve the above described
thermal properties.
In U.S. Patent 3,832,334, the problem of thermal resistance was solved by the addition of maleic
anhydrides and their substitution products. Additionally, it has been known in the prior art to include
cross-linking agents such as al|y|—2-cyanoacrylate or polymerizable acrylate esters to improve thermal
properties. However, none of the prior art has shown the improved thermal properties at 121°C (250°F)
that the instant invention is able to demonstrate. Thus, the need for such a useful adhesive is evident
and would prove useful in many applications.
The present invention provides an adhesive and sealant composition, which is normally liquid in
the uncured state, and upon cure exhibits a significantly improved hot strength at elevated temperatures
and improved resistance to thermal degradation. This composition comprises (a) at least one
polymerizable cyanoacrylate, (b) about 0.1% to about 10% by weight of the composition of at least one
difunctional or monofunctional polymerizable acrylate ester, (c) about 0.1 % to about 20% by weight of
the composition of an additive of formula:
10
15
20
30
-35
40
0
wherein R7 and R8 are alkyl, cycloalkyl, aralkyl or alkaryl,
(d) an anionic polymerization inhibitor; and optionally
(e) a free—radical polymerization inhibitor. .
The present invention solves the problems of the prior art adhesives, particularly their inability to 45
retain their structural integrity at elevated temperatures (hot strength), as well as their low resistance to
1 thermal degradation through heat aging. The compositions disclosed herein are useful for use with a
variety of surfaces, particularly steel aluminium, phenolics, epoxies, and thermoplastic materials. The
she|f—life stability and cure speed are excellent as well.
GB 2 100 273 A 2
10
15
20
25
30 structures:
The cyanoacrylate monomers useful in this invention are represented by the general formula:
CN
I
CH2=C—COOR |
wherein R is CH5 alkyl, cycloalkyl, alkenyl, cycloalkenyl, phenyl or a heterocyclic radical. The preferred
monomer which conforms to the general formula is ethyl cyanoacrylate, but a mixture of monomers of
the above formula can be used.
The polymerizable acrylate ester monomers useful in this invention may be mono- or
polyfunctional, or a mixture of both and conform to the general formulas:
CH2=C——COOR2 N
l
R1
wherein R‘ is H, CH3 or lower alkyl, R2 is H, or an alkyl, alkoxy, cycloalkyl, alkenyl, aralkyl, aryl, alkaryl or
aryloxy group; or
111. '
R3 R3
I I
R50 (CH2)m c c—-- o R5
R i23
wherein R3 is H, C,_4 alkyl or hydroxyalkyl or R5OCH2——; R5 is H, halogen or C,_4 alkyl; R4 is H, OH or
R5O——; R5 is CH2=CH°C=O; m is an integer, preferably 1 to 8; k is an integer, preferably 1 to 20; and p is
0 or 1.
There must be at least one acrylate ester monomer present in the composition of the invention,
generally in the amount of about 0.1% to about 10% by weight of the total composition. The preferred
amount is about 1 to about 5%, most preferably about 1% by weight.
Among the monofunctional polymerizable acrylate ester monomers (formula II) which are useful,
are hydroxyethyl methacrylate, hydroxypropyl methacrylate, isobornyl methacrylate, methyl
methacrylate, tetrahydrofurfuryl methacrylate, and butyl methacrylate, hydroxyethyl, hydroxypropyl and 20
methyl methacrylate and allyl methacrylate being preferred. ,
The polymerizable polyacrylate esters utilized in accordance with the invention and corresponding
to the above general formula lll are exemplified by, but not restricted to, the following materials:
diethylene glycol dimethacrylate, triethylene glycol dimethacrylate, tetraethylene glycol dimethacrylate,
dipropylene glycol dimethacrylate, di-lpentamethylene glycol) dimethacrylate, tetraethylene diglycerol
diacrylate, diglycerol tetramethacrylate, tetramethylene dimethacrylate, ethylene dimethacrylate,
neopentyl glycol diacrylate and trimethylol propane triacrylate. Of these, the preferred monomers are
triethylene glycol dimethacrylate and polyethylene glycol dimethacrylate.
Another ingredient essential to the present invention is an additive having one of the following
10
-P
15
25
30
O
N-R -N
N-R7 -R -N
O O
The nature of R7 and R5 is not critical for purposes of this invention and may be any organic radical
which does not contain any group which will adversely affect the composition for purposes disclosed
herein. Most commonly, R7 and R8 are selected from the group consisting of alkyl, cycloalkyl, aralkyl,
.§._____________________________________________________________
10
15
20
25
30
35
40
45
50
55
GB 2 100 273 A
alkaryl, aryl, aryloxy, alkoxy, any of which may be exceptionally large radicals; e.g., containing up to
about 200 carbon atoms or more; preferably they will contain from 6 to about 100 carbon atoms, most
preferably, 6 to 50 carbon atoms.
It has been found that resistance to thermal oxidative degradation is improved if R7 or R3 is
aromatic; however, this is not required for the general improvement of this invention to be realized. It
will, of course, be understood that both R7 and R3 can consist of relatively complicated moieties,
provided only that they do not contain functionality which interferes with the performance of the
additive for its intended purposes. The useful concentration range for this additive is about 0.1 to about
20%, preferably about 1 to about 5% by weight of the total composition and more preferably about 2%.
Without wishing to be bound by any one theory, it is believed that the improved hot strength
properties obtained from the compositions of the invention are a result of the unique combination of the
above maleimides with the polymerizable acrylate esters, both of which are vital constituents in the
cyanoacrylate adhesive composition. Theoretically, the maleimide additive reacts with the
cyanoacrylate monomer during polymerization. The maleimide ring opens to participate in the reaction
and grafts to the cyanoacrylate chain during its polymerization.
As referred to above it is not the intention that the invention should be bound by any particular
chemical theory, but it is believed that as the cyanoacrylate cures, the maleimide is incorporated into
the cyanoacrylate chain. Subsequent elevated temperatures are believed to induce a second stage of
polymerization between the grafted maleimides, which have unsaturated sites, and the acrylic ester
monomer. Cross—linkages are thus formed. The superior ability to maintain structural properties, such as
tensile strength, at temperatures of 121°C (250°F) or more for long periods of time, as well as to resist
the general effects of thermal aging, is attributed to this unique interaction.
Generally, the amount of the maleimide additive to be used is about 0.1 to about 20% by weight
of the composition, but the preferred amount is about 1 to about 5%, the most preferred amount being
2%. Amounts of about 2% or less readily dissolve at room temperature into the cyanoacrylate and
acrylic ester monomer. Above this amount, the additive may remain in suspension in the liquid
composition, still serving its function and producing the desired properties, however.
Among the maleimide additives preferred is the following structure:
0 0
where R3 is a phenylene group. This compound is manufactured by E. l. DuPont de Nemours Er Co.,
under the trade name HVA.
It is important to maintain proper stability of the composition without losing the advantage of fast
cure. The stability can be controlled by the use of known inhibitors of anionic polymerization.
Standard acidic gases, such as sulphur dioxide, sulphur trioxide and nitric oxide, can be
incorporated as conventional inhibitors of anionic polymerization. However, it is preferred that a
combination of sulphur dioxide and an acid selected from sulphonic acids, phosphorus acids,
phosphonic acids, and carboxylic acids, with a PKa range of about ~12 (negative twelve) to about 7
(seven) be used. This inhibiting system is disclosed in United States Patent Application, Serial No.
06/160,512,filed June 18, 1980. The most preferred components of the combination are sulphur
dioxide with methane sulphonic acid, both present in the range of about 0.005 to about 10% by weight
of the composition, but most preferably in the-range of about 0.005 to about 0.1%. The preferred
proportion of $02 to methane sulphonic acid is 20:50.
It is optional, but recommended, that an inhibitor of free-radical polymerization, selected from
hydroquinones, benzoquinones, naphthoquinones, phenanthraquinones, anthraquinones, and
substituted derivatives of any of the foregoing be incorporated into the adhesive as well. Hydroquinone
is the most preferred.
Generally, the amount of such inhibitors is about 0.17 to about 10% by weight of the composition
0.17 to 5% being preferred, and 0.95% being most preferred.
Other agents such as thickeners, plasticizers and diluents are also known in the art and may
advantageously be incorporated where functionally desirable, provided only that they do not interfere
with the functioning of the vital additives for their intended purposes. The instant compositions exhibit
good shelf—|ife stability, e.g., they normally remain liquid at room temperature in the uncured state. This,
of course, can be determined by simple experimentation.
The following examples are given to demonstrate the compositions within the scope of the
invention disclosed herein. These examples are not intended to be limitations on the scope of the
invention.
3
10
15
20
25
30
35
40
45
50
55
4 ‘ - GB2100273A‘4
Below is a Table of the ingredients in each of the compositions used in the Examples. All
percentages are by weight based on the amount of ethyl cyanoacrylate, which comprises the rest of the
adhesive composition.
TABLE I
Compositions
Additives (control) (control) (controls)
' % by weight A *3 c *0 E F G
HVA -—— — 2% 2% — 4% 2%
allyl meth-
acrylate — — 1% 1% 2% -—— 5%
inhibitors 0.2% 0.2% 0.2% 0.2% 0.2% 0.2% 0.2%
5 *Compositions B & D had approximately 5% thickening agents to increase their Brookfield viscosity to 5
200 cps at 25°C, using a No. 2 spindle.
EXAMPLE 1
Hot strengths of control composition A and B from Table l were measured at 121 °C (250°F). Grit-
blasted and solvent washed steel laps were used to prepare lap shear samples with these compositions
10 and the samples were then aged and tested at 121°C (250°F). The lap shear samples were allowed to 10
cure for 24 hours at room temperature before heat aging.
After 1 hour, the lap shear tensile strength of composition A was 1300 psi. After 25 hours, the
strength dropped to 700 psi, and after 48 hours, the strength was 650 psi.
Composition B was similarly tested and the results are tabulated in the Table below:
5
GB 2 100 273 A
xmm XLN XON RLN mam
$3 o\K—
BE 03. BB 63 58 EN:
mv_>> v_>> mm: mm: mm: m: 6.28
N _ mm mv em _ .gEo~
F_OO._
co_E.3o._ fimcea .N.\_mQ I zooms U0 _.N— ..m U9wm._.
Bum Bow
mv_>>
M
rooms on _.N— ._.< ozm_o< mmE< I._.0ZmE._.m ._.OI
__w4mE 2583 me. 8 mwofi ucm ¢ m_aEmxm: wo>_wm.£.m rm Sta 8 mfimcmbm «oz 9: 8 zowtmaeoo m :5: Eflmqam w_ EmEm>o.aE_ mg...
.$_:8oa 595nm ..o_._ m__m:B _o =o_E32 Em__moxm w>m; _._o_Em>:_ :58... m5 3 w:o:m_:E._o.. m5 8.: m.2mo::.._ Eng m>onm 9:.
$8 5% .38 xvv. .35.
38. Ba: Bot 39.: 89:
80V $8 $8 $3 gmm .x§ gov
\oNm_. B2: B3: 3%. >52 39: So: ,
8.; x; mm... mm: mm: mm... EI
N _. 8 E 3 vm w
_o:.:oo .._:>> uwaaeoo mm :o_Em..mm_ Smcmbm .x
Coments go here:
- Log in to post comments