Thermally Stable Nitrocellulose Emulsions, Solutions and Coatings
Folder:
Year:
Abstract:
Emulsions, solutions and coatings comprising nitrocellulose are stabilized against yellowing on thermal aging when treated with boric acid or borax or hydrogen peroxide or 0.1 to 0.5% by weight of a chlorinated hydantoin such as 1,3-dichloro 5,5-dimethylhydantoin (DCH). Boric acid or borax may be added as aqueous solutions during nitrocellulose manufacture. Combined treatments and/or additions of boric acid and DCH give optimum stabilization against yellowing.
Type of document:
Language:
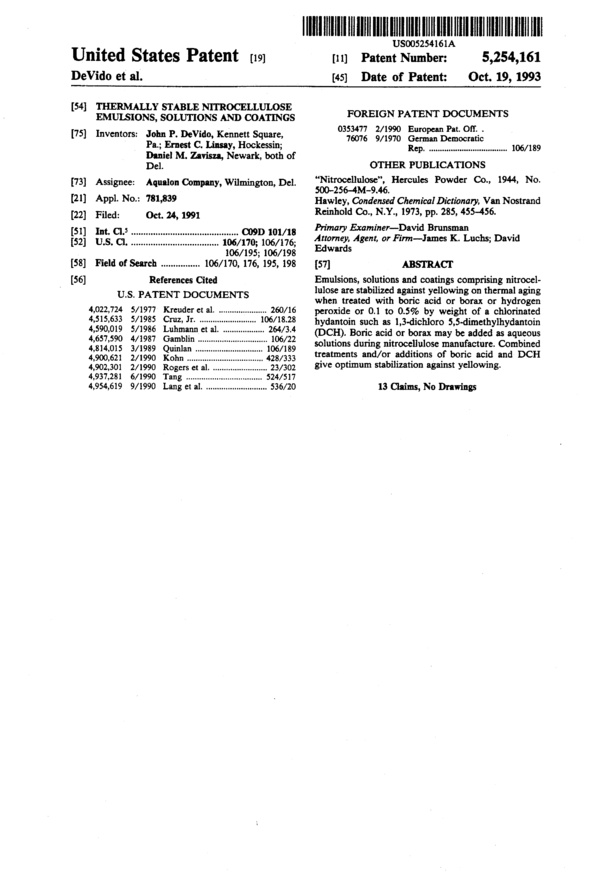
_ US005254161A
Umted States Patent [19] an Patent Number: 5,254,161
DeVido et al. [45] Date of Patent: Oct. 19, 1993
[54] THERMALLY STABLE NITROCELLULOSE
[75]
[73]
[21]
[22]
[51]
[52]
[58]
[56]
EMULSIONS, SOLUTIONS AND COATINGS
Inventors: John P. DeVido, Kennett Square,
Pa.; Ernest C. Linsay, Hockessin;
Daniel M. Zavisza, Newark, both of
Del.
Assignee: Aqualon Company, Wilmington, Del.
App]. No.: 781,839
Filed: Oct. 24, 1991
Int. Cl.5 .......................................... .. GED 101/18
U.S. Cl. .................................. .. 106/170; 106/176;
106/195; 106/198
Field of Search .............. .. 106/170, 176, 195, 198
References Cited
U.S. PATENT DOCUMENTS
4,022,724 5/1977 Kreuder et al. .................... .. 260/16
4,515,633 5/1985 Cnlz, Jr. ........... .. . 106/18.28
4,590,019 5/1986 Luhmann et al. 264/3.4
4,657,590 4/1987 Gamblin . . . . . . . . . . . . . . . . . . .. 106/22
4,814,015 3/1989 Quinlan ..... .. 106/189
4,900,621 2/1990 Kohn ............. .. 428/333
4,902,301 2/ 1990 Rogers et al. .... .. 23/302
4,937,281 6/1990 Tang .............. .. 524/517
4,954,619 9/1990 Lang et al. ........ .. 536/20
FOREIGN PATENT DOCUMENTS
0353477 2/1990 European Pat. Off. .
76076 9/ 1970 German Democratic
Rep. .................................. .. 106/189
OTHER PUBLICATIONS
“Nitrocellulose”, Hercules Powder Co., 1944, No.
500—256—4M—9.46.
Hawley, Condensed Chemical Dictionary, Van Nostrand
Reinhold Co., N.Y., 1973, PD. 285, 455-456.
Primary Examiner—David Brunsman
Attorney, Agent, or Finn——James K. Luchs; David
Edwards
[57] ABSTRACT
Emulsions, solutions and coatings comprising nitrocel-
lulose are stabilized against yellowing on thermal aging
when treated with boric acid or borax or hydrogen
peroxide or 0.1 to 0.5% by weight of a chlorinated
hydantoin such as 1,3-dichloro 5,5-dirnethylhydantoin
(DCH). Boric acid or borax may be added as aqueous
solutions during nitrocellulose manufacture. Combined
treatments and/or additions of boric acid and DCH
give optimum stabilization against yellowing.
13 Claims, No Drawings
7 5,254,161
1
THERMALLY STABLE NITROCELLULOSE
EMULSIONS, SOLUTIONS AND COATINGS
FIELD OF THE INVENTION
This invention relates to nitrocellulose products used
in the form of emulsions and solutions for coatings. In
particular the invention relates to nitrocellulose emul-
sions and solutions for coatings which exhibit reduced
yellowing.
BACKGROUND OF THE INVENTION
Nitrocellulose was once widely used for automotive
finishes. Henry Ford had said “Give them any color
they want as long as it’s black.” This was significant
since a black nitrocellulose car finish did not show yel-
lowing on aging. It was this problem with yellowing
which led to the phase out of nitrocellulose for auto
finishes as consumers demanded more than basic black.
Yellowing continues to be a problem in nitrocellulose
uses for furniture lacquers and clear nail polish.
Technological advances continued even though ni-
trocellulose yellowing was not addressed. The develop-
ment of an improved nitrocellulose manufacturing pro-
cess is described in U.S. Pat. No. 4,590,019. A water
based nitrocellulose spray paint is described in Euro-
pean Patent Application 0 353 477 Al. U.S. Pat. No.
4,900,621 discloses pinhole-free nitrocellulose coatings.
U.S. Pat. No. 4,954,619 discloses an improved film
forming nitrocellulose nail polish.
U.S. Pat. No. 4,022,724 discloses the use of bis-
hydroxyphenyl-3-alkanes for prevention of yellowing.
It would appear from U.S. Pat. No. 4,937,281 that a
need existed for non-yellowing clear acrylate coatings
similar to the unresolved problem with nitrocellulose
yellowing.
Thus, an unsatisfied need existed prior to the present
invention to prevent yellowing when nitrocellulose was
manufactured and used in the form of solution, emulsion
or coating.
SUMMARY OF THE INVENTION
It is an object of the invention to provide a nitrocellu-
lose composition in the form of a coating solution or
emulsion, characterized in that the composition exhibits
reduced yellowing during aging wherein the composi-
tion comprises an effective amount of hydrogen perox-
ide or boric acid or an alkali metal salt of boric_acid
and/or a chlorinated hydantoin having the following
structure:
O=0
wherein X can be C1 or Br,
where R1 and R2 can be hydrogen or methyl groups.
The preferred compound (l,3-dichloro-5,5-dimethyl-
hydantoin) has the structure:
10
l5
20
25
30
35
45
55
65
2
ii
Cl
\ c
N’ \N—Cl
I
Me-C [
/ \
Me C
ll
0
A preferred nitrocellulose composition for furniture
lacquer or clear nail polish coating comprises at least
one of:
(a) boric acid or borax; and
(b) l,3—dichloro-5,5-dimethylhydantoin (DCH);
wherein a coating composition or a coating containing
one or both of these exhibits lower yellowing on ther-
mal aging.
It is a further object of the invention to provide a
manufacturing process for improving the thermal stabil-
ity of a nitrocellulose composition comprising the steps:
(1) producing an unstabilized nitrocellulose in an aque-
ous medium;
(2) mixing the nitrocellulose in an aqueous medium
containing dissolved boric acid or borax (Na2B203.lO
H20) for a time sufficient for the nitrocellulose to
absorb a stabilizing amount of boric acid or borax;
and
(3) recovering a stabilized nitrocellulose product suit-
able for preparing a furniture lacquer or clear finger
nail polish.
Combined treatments and/or additions of borax and
DCH are preferred for optimum benefit against yellow-
mg.
DETAILED DESCRIPTION OF THE
INVENTION
U.S. Pat. No. 4,937,281 describes the use of ultravio-
let stabilizers to prevent yellowing in acrylate coatings.
While it would appear that such technique might simi-
larly apply to nitrocellulose yellowing, it was in fact
discovered that thermal stability was the major problem
requiring a solution. Finding such a solution was the
advance in the state of the art provided by the inven-
tion.
It was indeed a surprising and unexpected result to
find how simply and effectively both classes of addi-
tives worked, and further that boric acid and borax
could be incorporated during the nitrocellulose manu-
facturing process. This advance is further significant in
view of the length of time that this yellowing problem
had existed without clues to any solution prior to the
present invention.
It has been discovered that activity for prevention of
yellowing can vary as structural changes are made to
the hydantoin,’ for example
Most Active
0
ll
Cl c
\ / \
1“ ‘.“‘°'
Me—C C
/ \\
0
Me
Less Active
5,254,161
3
-continued
0
II
c
\ / \
N N—Br
I I
Me—C C
/ \\
0
Cl
Me
While l,3-dichloro-5,5-dimethylhydantoin (DCH) is
preferred, other suitable compounds include N-
chlorosuccinimide. Effective amounts of additions are
-from 0.2 to 5.0% by weight based on the weight of the
nitrocellulose. Preferred amounts are from 0.4 to 2.0%
by weight.
While hydrogen peroxide solutions and boric acid
and borax (Na; B2 03 . l0 H20) are preferred as water
soluble additions, other alkali metal salts such as potas-
sium borate and lithium borate could be employed.
Effective amounts of additions are from 0.1 to 5.0% by
weight based on the weight of nitrocellulose. Preferred
amounts are from 0.8 to 1.0% by weight.
Stabilized nitrocellulose can be produced by a novel
manufacturing process wherein water soluble boric acid
and/or borax can be added during a washing step con-
current with nitrocellulose manufacture.
A preferred process involves the steps:
(1) producing a fibrous form of unstabilized nitrocellu-
lose in an aqueous medium;
(2) mixing the nitrocellulose with a solution of boric
acid during a washing step and allowing the nitrocel-
lulose to equilibrate at 40° C. for at least 30 minutes;
and
(3) decanting the now stabilized nitrocellulose to pro-
duce a product slurry suitable for further treatment
and formulation to prepare a lacquer or a clear finger-
nail polish.
During step (2) approximately 1 liter of a 0.5% by
weight boric acid solution is used to treat 450 g of solid
nitrocellulose. While elevated temperature might in-
crease the rate of adsorption of the boric acid onto the
nitrocellulose, this is not a recommended technique due
to potential exotherrnal excursions which have been
known to occur during nitrocellulose manufacturing
processes.
The invention which has applicability for industrial,
home and personal use coatings is illustrated by the
following examples.
EXAMPLE l
An aqueous nitrocellulose/acrylate emulsion was
prepared according to the techniques described in U.S.
Pat. Nos. 3,953,386 and 4,011,388 except that sodium
hydroxide was used in place of sodium bicarbonate.
With and without additions according to the invention,
control and experimental formulations were drawn
down onto white glass plates to prepare test coatings.
These coatings were aged at 150° F. (66° C.), and yel-
lowing was followed by periodically measuring Yel-
lowness Index (Y I). Table 1 contains comparative re-
sults of the testing.
TABLE 1
150° F. 150° F.
Addition % Addition Yl Initial Yl 24 Hrs. Y] 48 Hrs.
Control — 6.66 22.43 26.86
DCH 0.2 2.43 10.46 12.27
Boiic Acid 0.5 4.92 3.02 10.09
l0
15
20
25
30
3.5
40
45
50
55
65
4
TABLE l-continued
150' F. 150' F.
Addition % Addition Yl Initial YI 24 Hrs. Yl 48 Hrs.
DCH + Borax 0.2 + 0.5 3.90 6.05 8.09
As shown, the best protection against yellowing on
thermal aging involved the DCH and boric acid combi-
nation.
EXAMPLE 2
A solvent nitrocellulose lacquer was prepared using
the following formulations:
Nitrocellulose i see. (IPA wet) 750 g
Ethanol 2B 107 g
Butnnol 172 g
Xylene 770 g
Methyletliylketone (MEK) 259 g
Butyl acetate 548 g
Exxate ® 600 (Exxon Chemicals) 51 g
Dried costings were thermally aged by first being
tested for 44 hours at 1500' F. followed by 47 hours at
l800° F. Results are shown in Table 2.
TABLE 2
Y1 After
Addition Initial YI Therm. Aging
Control 2.69 8.09
DCH 1.32 5.58
As shown, DCH gives superior protection under
thermal aging.
EXAMPLE 3
Example 1 was repeated except that either borax or
hydrogen peroxide was substituted for boric acid. Simi-
lar results were obtained, but hydrogen peroxide gave
slightly less yellowing protection than DCH.
EXAMPLE 4
Example 1 was repeated except that the water wet
nitrocellulose was manufactured with different viscos-
ity, which varied from 1 sec. to 5 sec. Equivalent pre-
vention of yellowing was obtained with DCH, borax or
boric acid and combinations thereof. Slightly poorer
protection was provided when hydrogen peroxide was
used in from 1% to 5% by weight based on the weight
of nitrocellulose.
EXAMPLE 5
Example 1 was repeated except that a bromo-
chlorohydantoin was substituted for DCH. This was
less effective at equivalent addition levels to DCH, but
it was suitable for providing significant protection from
thermal aging yellowing for either an emulsion or a
coating.
COMPARATIVE EXAMPLE 6
Materials tested in the same manner as DCH which
proved to be ineffective against yellowing on thermal
aging were:
N-bromosucciriimide, N-bromoacetamide, trichloro-
melamine, trichloroisocyanuric and, N-bromophthali-
mide, azobisisobutyronitrile, benzophenone, dicumyl-
peroxide, mixture of para and meta isomers of alpha,
i254J6l
5
alpha’-bis(t-butylperoxy)diisopropylbenezene,
peroxide, and t-butylhydroperoxide.
EXAMPLE 7
Example 3 was repeated except that benzoyl peroxide
MEK
was substitited for hydrogen peroxide. Similar reduced .
yellowing was observed.
What is claimed is:
1. A nitrocellulose composition in the form of a coat-
ing, solution or emulsion, characterized in that the com-
position exhibits reduced yellowing during aging
wherein the composition comprises nitrocellulose and
an effective amount of one or more of borax or a chlori-
nated hydantoin having the following structure:
0
II
C
X-l'|~I/ ‘1'~1—c1
R1‘-C C
I ll
R2 0
wherein X can be C1 or Br, and where R1 and R2 can be
hydrogen or methyl groups.
2. The nitrocellulose composition of claim 1 where
the composition includes the chlorinated hydantoin that
is 1,3-dichloro-5,5-dimethylhydantoin (DCH) with the
structure:
added in an amount of 0.2 to 5.0% by weight based on
the weight of nitrocellulose.
l0
15
20
25
30
35
45
50
55
65
6
3. The composition of claim 2, further characterized
in that the composition contains 0.4 to 2.0 weight per-
cent DCH based on the weight of nitrocellulose.
4. The composition of claim 1 where boric acid or
borax are added to an aqueous nitrocellulose system.
5. The composition of claim 1, further characterized
in that the composition contains 0.01 to 0.50 weight
percent boric acid or borax based on the weight of
nitrocellulose. .
6. A process for improving the thermal stability of a
nitrocellulose composition comprising the steps
(1) producing an unstabilized nitrocellulose in an
aqueous medium,
(2) mixing the nitrocellulose in an aqueous medium
containing dissolved boric acid or borax for a time
sufficient for the nitrocellulose to adsorb a stabiliz-
ing amount of boric acid or borax for at least 30
minutes at 40° C.,
(3) recovering a stabilized nitrocellulose product
suitable for preparing a coating composition.
7. A nitrocellulose protective or decorative coating
comprising nitrocellulose and an organic solvent, char-
acterized in that the nitrocellulose is stabilized against
yellowing on thermal aging with DCH or borax or
hydrogen peroxide.
8. The coating of claim 7 where the nitrocellulose is
contained in a nitrocellulose lacquer.
9. The coating of claim 8 containing from 1 to 5% by
weight hydrogen peroxide based on the weight of nitro-
cellulose.
10. The coating of claim 7 where the nitrocellulose is
contained in a nitrocellulose/acrylate or nitrocellulose
lacquer emulsion.
11. The coating of claim 7 where the nitrocellulose is
contained in a nail polish composition.
12. The coating of claim 7 containing from 0.8 to
1.0% by weight borax or boric acid based on the weight
of nitrocellulose.
13. The coating of claim 7 containing from 0.4 to
2.0% by weight DCH based on the weight of nitrocellu-
lose.
1 3 I 3 t
Coments go here: