3DFIX
Impregnating resin for 3D printed parts
AFINITICA® 3D Fix is a cyanoacrylate adhesive designed specifically for the impregnating and curing within 3D printed gypsum parts. Due to its low viscosity and high penetration depth, it wicks efficiently inside the parts increasing their robustness and durability.
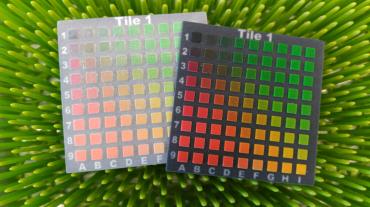
Furthermore, the resin has the effect of enhancing colours that appear more intense and vibrant after impregnation. The treatment achieves a professional, high quality gloss finish. One product achieves enhanced properties and finish to serve the needs in 3D printing.

AVAILABLE FORMATS
High impregnation capability
Drying in 24h
Glossy finish
Can be used as a coating
Enhance colours
Odourless and low blooming
Non-irritant, label-free
Transparent and easy to use
• Impregnating 3D printed parts
• Strengthening printed parts
• Glossy finish coating