PHENYL ETHANOL DERIVATIVES
Year:
Type of document:
Language:
United States Patent [191 I
Allais et al.
1111 3,892,773
[451 July 1,1975
1541 2-PHENYL ETHANOL DERIVATIVES
[75] Inventors: Andre Allais, Les Lilas; Jean Meier,
La Varenne Saint-Hilaire; Jacques
Dube, Eaubonne, all of France
[73] Assignee: Roussel Uclaf, Paris, France
[22] Filed: May 21, 1973
[21] Appl. No.: 362,172 '
[30] Foreign Application Priority Data
June 1, 1972 France ............................ .. 72.19690
[52] U.S. Cl....260/327 TH; 260/295.5 R; 260/345.8;
260/345.9; 424/266; 424/275; 424/283
[51] Int. Cl. ...................... .. C07d 65/04; C07d 7/04
[58] Field of Search ..... .. 260/327 TH, 345.8, 345.9,
260/618 D, 488 CD; 424/275, 283, 317, 333,
343
[56] References Cited
UNITED STATES PATENTS
3,452,079 6/1969 Shen ................................. .. 260/469
3,669,973 6/1972 Borck .......................... .. 260/293.73
FOREIGN PATENTS OR APPLICATIONS
2,054,501 4/1971 France
Primary Examiner—Henry R. Jiles
Assistant Examiner-—C. M. S. Jaisle
Attorney, Agent, or Firm——Hammond & Littell
[57] ABSTRACT
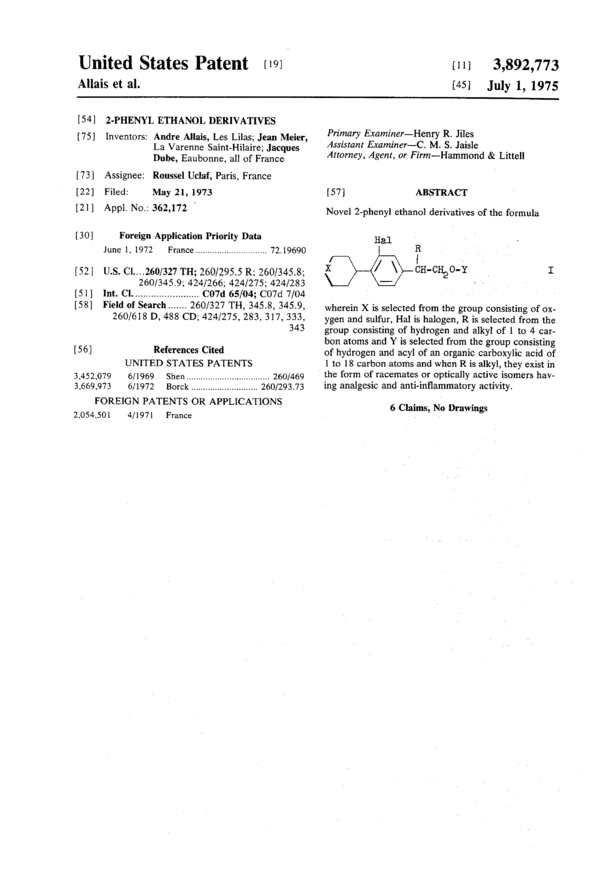
Novel 2-phenyl ethanol derivatives of the formula
Hal
R
9 22,120., I
wherein X is selected from the group consisting of ox-
ygen and sulfur, Hal is halogen, R is selected from the
group consisting of hydrogen and alkyl of 1 to 4 car-
bon atoms and Y is selected from the group consisting
of hydrogen and acyl of an organic carboxylic acid of
1 to 18 carbon atoms and when R is alkyl, they exist in
the form of racemates or optically active isomers hav-
ing analgesic and anti-inflammatory activity.
6 Claims, No Drawings
3,892,773
1
Z-PHENYL ETHANOL DERIVATIVES
OBJECTS OF THE INVENTION
It is an object of the invention to provide the novel
2-phenyl-ethanol derivatives of formula I.
It is another object of the invention to provide a
novel process for the preparation of the compounds of
formula I.
It is an additional object of the invention to provide
novel analgesic and anti-inflammatory compositions
and to provide a novel method of relieving pain and in-
flammation in warm-blooded animals.
These and other objects and advantages of the inven-
tion will become obvious from the following detailed
description.
THE INVENTION
The novel 2-phenyl-ethanols of the invention have
the formula
Hal R
\‘j._cH-cngo-Y I
wherein X is selected from the group consisting of oxy-
gen and sulfur, Hal is halogen, R is selected from the
group consisting of hydrogen and alkyl of 1 to 4 carbon
atoms and Y is selected from the group consisting of
hydrogen and acyl of an organic carboxylic acid of 1 to
18 carbon atoms and when R is alkyl, they exist in the
form of racemates or optically active isomers.
Examples of suitable acids are alkanoic acids, such as
formic acid, acetic acid, propionic acid, butyric acid,
isobutyric acid, valeric acid, isovaleric acid, trimethyl
acetic acid, caproic acid, B-trimethylpropionic acid,
heptanoic acid, caprylic acid, pelargonic acid, capric
acid, undecylic acid, lauric acid, myristic acid, palmitic
acid and stearic acid; alkenoic acids such as undecy-
lenic acid and oleic acid; cycloalkyl carboxylic acids
such as cyclopentyl carboxylic acid, cyclopropyl car-
boxylic acid, cyclobutyl carboxylic acid and cyclohexyl
carboxylic acid; cycloalkyl alkanoic acids such as cy-
clopentyl acetic acid, cyclohexyl acetic acid, cyclopen-
tyl propionic acid and cyclohexyl propionic acid; ary-
lalkanoic acids such as phenylacetic acid and phenyl-
propionic acid; aryl carboxylic acids such as benzoic
acid and 2,4-dinitrobenzoic acid; phenoxy alkanoic
acids such as phenoxyacetic acid, p-chlorophenoxy
acetic acid, 2,4-dichlorophenoxy acetic acid, 4-tert.-
butylphenoxy acetic acid, 3-phenoxy propionic acid
and 4-phenoxy butyric acid; heterocyclic carboxylic
acids such as furane-2-carboxylic acid, 5-tert.-
butylfurane-2-carboxylic acid, 5-bromofurane-2-
carboxylic acid and nicotinic acids; ,8-keto-alkanoic
acids such as acetylacetic acid, propionylacetic acid
and butrylacetic acid; amino acids such as die-
thylaminoacetic acid and aspartic acid.
Among the preferred compounds of formula I, R is
ethyl or propyl, most preferably methyl; Hal is fluorine
or bromine and most preferably chlorine; and Y is hy-
drogen or lower alkanoic acids such as formic, acetic,
propionic, butyric or isobutyric acids.
The novel process of the invention for the prepara-
tion of the compounds of formula I comprises reacting
a compound of the fonnula
5
10
15
20
25
30
35
40
45
50
55
60
65
2 .
Hal T
/ \i/>—f3H—CH20—Y
cg
wherein X, R and Hal have the above definitions and
Z is selected from the group consisting of hydrogen and
alkyl of l to 5 carbon atoms with a reducing agent to
form the corresponding alcohol of formula I wherein Y
is hydrogen and if desired the alcohol is esterified to
form the corresponding ester.
The preferred reducing agents are lithium aluminum
hydride or diborane and the preferred esterification are
the acid chloride or acid anhydride of the acid Y-OH
and the reaction is effected in the presence of a tertiary
base such as pyridine.
The acids and lower alkyl esters of formula II may be
prepared by the process described in Belgium Pat. No.
753,539. When R is alkyl of I to 4 carbon atoms, the
compounds of formula II may be in the form of race-
mates or optically active isomers obtained by resolving
the acid of formula II with an optically active base by
known means. This produces the corresponding race-
mates or optically active isomers of formula I.
The analgesic and anti-inflammatory compositions of
the invention are comprised of a compound of formula
I and a pharmaceutical carrier. The compositions may
be in the form of injectable solutions or suspensions,
tablets, capsules, gelules, drinkable solutions or suspen-
sions, suppositories, pomades, creams or topical pow-
ders prepared in the usual fashion.
The compositions may be used in therapy for the
treatment of inflammatory maladies such as rhumatis-
mal affections, arthroses, lumbago, zoster and also as
a complemetary treatment for infections and feverish
states as well as the treatment of muscular, articular or
nervous pain, tooth aches and migraines.
The novel method of relieving pain and inflammation
in warrn-blooded animals comprises administering to
warrn-blooded animals an effective amount of at least
one compound of formula I. The compounds may be
administered orally, parenterally or rectally or topically
to the skin or mucous membranes. The usual daily dose
is 0,4 to 4 mg/kg depending upon the product and
method of administration.
In the following examples there are described several
preferred embodiments to illustrate the invention.
However, it should be understood that the invention is
not intended to be limited to the specific embodiments.
EXAMPLE 1
2—methyl-2-[4’-(4"-tetrahydropyranyl)-3 ’-chloro-
phenyl]-ethanol
A mixture of 2.4 g of lithium aluminum hydride in 20
ml of tetrahydrofuran was heated to reflux and then, a
solution of 2.4 g of 2-methyl-2-[4’-(4"-
tetrahydropyranyl)-3 ’-chloro-phenyl]-acetic acid (pre-
pared as in Belgium Pat. No. 753,539) in 20 ml of tetra-
hydrofuran was added dropwise. The mixture was
stirred for 2 hours and then cooled. 25 ml of isopropa-
nol and 20 ml of an aqueous solution saturated with
ammonium chloride were slowly added thereto and the
mixture was filtered. The filtrate was decanted to re-
cover the organic phase which was washed with water
until the wash water was neutral. The organic phase
3,892,773
3
was evaporated to dryness under reduced pressure to
obtain an 82 percent yield of 2-methyl-2-[4’—(4"-
tetrahydropyranyl )-3 ’-chloro-phenyl ] -ethanol which
was soluble in the usual organic solvents and insoluble
in water.
Analysis: CHH,,.ClO2; molecular weight = 254.75
Calculated: %C 66 %H 7.51 %Cl 13.9
Found: 66 7.2 13.9
%O 12.56
12.8
The IR spectrum was in accord with the indicated
structure.
EXAMPLE 2
Acetate of
2-methyl-2-[4 ’-( 4 ' ’-tetrahydropyranyl)-3 ’-
chlorophenyl]-ethanol
3 ml of acetyl chloride were added dropwise to a mix-
ture of 7.05 g of the product of Example 1 in 21 ml of
pyridine cooled to 0°C and the mixture was stirred for
one hour at room temperature. The excess acetyl chlo-
ride was destroyed by the addition of chopped ice and
the mixture was then poured into a solution of 23 ml of
concentrated hydrochloric acid in 200 ml of water and
ice. The mixture was extracted with ethyl ether and the
ether, extracts were washed with water until the wash
waters were neutral and evaporated to dryness under
reduced pressure..The residue was purified by chroma-
tography over silica gel and with a 7:3 chloroformethyl
acetate eluantand evaporation of the solvent resulted
in a 63 percent yield of the acetate of 2-methyl-2-[4’-
(4’ ’-tetrahydropyranyl )-3 ’-chloro-phenyl ] -ethanol.
Analysis: C,’.;H2zClO;;; molecular weight = 297.80
Calculated: %C 64.53 %H 7.44 1
Found: _ 64.7 7.1 1
The IR spectrum is in accord with the indicated struc-
ture.
EXAMPLE 3
2-methyl-2-[4 ’-( 4 ’ ’—tetrahydrothiapyrany1 )-3 ’-chloro-
phenyl]ethan0l
A solution of 1.93 g of 2-methyl-2~[4’-(4”-
tetrahydrothiapyranyl)-3 ’-chloro-phenyl ]-acetic acid
in 90 ml. of tetrahydrofuran was added with stirring to
a refluxing mixture of 1.75 g of lithium aluminum hy-
dride in 70 ml of tetrahydrofuran and after 10 minutes
of reflux, another 1.75 g of lithium aluminum hydride
were added. Reflux was continued for 20 minutes and
the mixture was then cooled to about 0°C.
A mixture of 125 ml of 5 N hydrochloric acid and
100 g of ice was added with stirring followed by 80 ml
of ethyl ether and then sufficient sodium chloride for
saturation. The organic phase was decanted and the
aqueous phase was extracted with ethyl ether. The
combined organic phases were evaporated to dryness
under reduced pressure and the residue was purified by
chromatography over silica gel. The eluant was a 3-2
mixture of chloroform-acetone and evaporation of the
solvents gave a 75 percent yield of 2-methyl-2-[4’-(4”-
tetrahydrothiapyranyl )-3 ’-chloro-phenyl]-ethanol
10
15
20
25
30
35
40
45
50
55
60
65
4
which was insoluble in water and soluble in the usual
organic solvents.
Analysis: C,4H.,,Cl0S; molecular weight = 270.78
%C %H %Cl %S
Calculated: 62.09 7.07 13.09 1 1.84
Found: 621 7.2 13.3 11.4
The IR spectrum and the mass spectrum agreed with
the indicated structure.
PHARMACOLOGICAL STUDY
A. Anti-inflammatory Activity
The anti-inflammatory activity was determined by
the test of Jequier et al [Arch. Int. Pharrnacodyn, Vol.
152 (1954), p. 15] with groups of rats weighing about
150 g receiving a single injection of 1 mg of naphthoyl-
heparamine in the plantar aponeurosis of the hind paw
to provoke formation of inflammatory edema. The test
products were orally administered in an aqueous sus-
pension one hour before the irritant injection and the
volume of the paw was measured immediately before
the injection and 2 hours later. The increase in paw vol-
ume represents a measure of the degree of inflamma-
tion. The dose that reduced the degree of inflammation
by 40 percent of the controls was determined and is re-
ported in Table I as DA40.
B. Analgesic Activity
The test used was based on the fact noted by R.
Koster et al [Fed. Proc., (1959), Vol. 18, Page 412]
wherein the intraperitoneal injection of acetic acid
causes in mice characteristic repeated stretching and
twisting movements which can persist for more than 6
hours. Analgesics prevent or surpress this syndrome
which, therefore, can be considered as externalization
of a diffuse abdominal pain.
A solution of 0.6 percent of acetic acid in water con-
taining l0 percent arabic gum was used and the dose
which released thesyndrome under these conditions
was 0.01 ml/gm, that is 60 mg/kg of acetic acid. The
test compounds were administered orally % hour be-
fore the intraperitoneal injection of acetic acid, the
mice having fasted since the night before the experi-
ment. For each dose and for each control, which are
obligatory for each test, a group of 5 animals was used.
For each mouse, the stretchings were observed and
counted and then added for the group of 5 during a pe-
riod of 15 minutes starting immediately after the injec-
tion of acetic acid. The results in the following Table
are given as the dose which reduces by 50 percent the
number of stretching as compared to the controls
(DA5o)-
TABLE I
Compound of Example DA“, mg/kg DA-,0 mg/kg
1 0.05 20
2 12 20
3 0.065 8
The data of Table lshows that the compounds have
anti-inflammatory and analgesic activity with the prod-
ucts of Examples 1 and 3 having a particularly remark-
able anti-inflammatory activity.
' 3,892,773
5 6
Various modifications of the products and methods group consisting of hydrogen and alkyl of 1 to 4 carbon
of the invention may be made without departing from atoms and Y is selected from the group consisting of
the Splfll or scope thereof and ll: is to be understood hydrogen and acy] of a Iower alkanoic acid and when
glatdthe iii:/emion igitelnfiled 10 be limited 0111)’ as de' R is alkyl, they exist in the form of racemates or opti-
me in t e appen e c arms. 5 11 t- - _
We Claim Ca2.y:cc::;:::::r:f claim 1 wherein R is methyl.
1' A °°mp°”"d of the formula 3. A compound of claim 1 wherein Hal is chlorine.
Hal 4,1,. A compound of claim 1 which is 2-methyl-2-[4’-
R 0 10 (4 -tetrahydropyranyl)-3 -chloro-phenyl]-ethanol.
X / -\ E H II 5. A compound of claim 1 which is the acetate of 2-
- H-C-OZ methyl-2-
\L__. \c2 [ 4 '-(4’ ’-tetrahydropyranyl )-3 ’-chloro-phenyl]-ethanol.
6. A compound of claim 1 which is 2-methyl-2-[4’-
wherein X is selected from the group consisting of oxy- 15
Coments go here:
- Log in to post comments