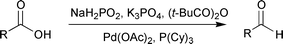New Pd-catalyzed selective reduction of carboxylic acids to aldehydes
New Pd-catalyzed selective reduction of carboxylic acids to aldehydes
Journal:
Year:
Abstract:
A catalyst generated in situ from palladium acetate and tricyclohexylphosphine efficiently catalyzes the reduction of carboxylic acids with sodium hypophosphite in the presence of pivalic anhydride to give aldehydes with high selectivity. The low cost and convenient handling of the reagents makes this process a valuable alternative to hydrogenations and metal hydride reductions.
DOI:
10.1039/B201577N
Type of document:
Language:
View Online
New Pd-catalyzed selective reduction of carboxylic acids to aldehydes
Lukas J. Gooßen* and Keya Ghosh
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, D-45470 Mülheim an der Ruhr,
Germany. E-mail: goossen@mpi-muelheim.mpg.de; Fax: +49-208-306-2985; Tel: +49-208-306-2392
Received (in Cambridge, UK) 12th February 2002, Accepted 1st March 2002
First published as an Advance Article on the web 21st March 2002
DOI: 10.1039/b201577n
Downloaded on 09 November 2010
Published on 21 March 2002 on http://pubs.rsc.org | doi:10.1039/B201577N
A catalyst generated in situ from palladium acetate and
tricyclohexylphosphine efficiently catalyzes the reduction of
carboxylic acids with sodium hypophosphite in the presence
of pivalic anhydride to give aldehydes with high selectivity.
The low cost and convenient handling of the reagents makes
this process a valuable alternative to hydrogenations and
metal hydride reductions.
836
The synthesis of aldehydes from carboxylic acid derivatives is
an important transformation often used in commercial and
laboratory scale organic synthesis.1 Several suitable reducing
agents have been reported for the selective reduction of acids,
esters, and amides such as lithium tris-tert-butoxyaluminium
hydride,2 aminoaluminium hydrides,3 DIBAL-H,4 and
NaAlH4.5 Alternatively, the sensitive and highly reactive acid
chlorides can be hydrogenated in a Rosenmund catalytic
reduction6 and related reactions using metal hydrides.7,8
However, in all these methods, the acid derivatives have to be
generated in an additional reaction step and the handling of the
metal hydrides requires extreme caution and rigorous exclusion
of air and moisture. Recently, Yamamoto et al. developed an
elegant process for the direct hydrogenation of carboxylic acids
to aldehydes by using pivalic anhydride to convert the acids in
situ into mixed anhydrides, and reducing these at high hydrogen
gas pressures.9,10 The mixed anhydrides are selectively hydrogenated at the sterically less demanding site, so that pivalic acid
is always produced as the by-product along with the desired
aldehyde.9 Pivalic anhydride itself is inert under these conditions. Although this reaction protocol is excellent for industrial
scale applications, the need for high-pressure equipment makes
it rather inconvenient for small-scale laboratory applications.
We herein present a practical and simple process for the
selective hydrogenation of carboxylic acids to aldehydes using
cheap and easy-to-handle aqueous sodium hypophosphite
solution as the reductant (Scheme 1).
In some initial experiments, we observed that in contrast to
other reducing agents such as silanes, boranes, metal hydrides,
or formates, hypophosphorous acid salts could selectively
reduce carboxylic anhydrides into aldehydes in the presence of
Pd–phosphine complexes. This was surprising since hypophosphites had been used to reduce aldehydes to alcohols in the
presence of Pd/C or Raney nickel.11 Intrigued by this observation, we studied the reduction of a mixture of benzoic acid 1a
and pivalic anhydride 2 in some detail, screening several Pd–
phosphine complexes under various conditions in order to
identify an efficient catalyst system (Scheme 1). Selected
results are shown in Table 1.
In good accordance with the findings described above,11 the
desired selectivity for the aldehyde 3a was achieved only with
Pd–phosphine complexes as catalysts (Entries 1–3, 7). Whenever this catalyst decayed during the reaction and non-ligated
palladium formed, the benzyl alcohol 5a was produced in
Scheme 1 Reduction of benzoic acid with sodium hypophosphite.
CHEM. COMMUN., 2002, 836–837
significant quantities (Entry 2). The addition of a mild base was
found to stabilize the palladium complexes and ensure a high
selectivity for the aldehyde versus the alcohol. In this respect,
potassium phosphate and sodium carbonate were most effective
(Entries 3–7). Besides sodium hypophosphite, other hypophosphite salts can be used (Entries 8 and 9) but did not appear
advantageous. When hypophosphorous acid itself was used as
the reducing agent in the absence of a base, no product was
formed (Entry 10).
Among all phosphines tested, the electron rich, sterically
demanding cyclohexylphosphine gave the best results (Entries
11–16). Among the palladium precursors, palladium acetate
was most effective (Entries 17–19).
The reaction proceeds best in THF as the solvent (Entries 7,
20–22) and the presence of water is beneficial (Entries 7, 23,
24). In the absence of water, the reaction is extremely slow,
probably due to the insolubility of the reducing agent. If too
much water is added, the hydrolysis of the anhydrides becomes
most prevalent, thus lowering the yield. The reaction is not
particularly sensitive towards oxygen; however, it is beneficial
Table 1 Optimization of the reaction conditions
Entry Catalyst
Base
Solvent
3a (%)
1
2
3
4
5
6
7
8a
9b
10c
11
12
13
14
15
16
17
18
19
20
21
22
23d
24e
5a (%)
Pd(OAc)2
—
THF
Coments go here:
- Log in to post comments