Soluble High Polymers from Allyl Methacrylate
Soluble High Polymers from Allyl Methacrylate
Journal:
Year:
Abstract:
Allyl methacrylate has been polymerized by free-radical methods and found to yield a soluble polymer in carbon tetrachloride, dioxane, and diallyl ether solutions. The overall rate equation in diallyl ether is Rp = k[ln]0.7[M]1.6. It is suggested that propagation and cyclization reactions proceed only via addition to the methacrylyl groups of the monomer. Some degradative chain transfer occurs with the allyl groups, and it is considered that the solvents may ensure the production of soluble polymers by reactions in which allyl–radical side chains are terminated without crosslinking.
DOI:
10.1002/pol.1968.150061105
Type of document:
Language:
JOURNAL OF POLYMER SCIENCE: PART A-1
VOL. 6, 3007-3013 (1968)
Soluble High Polymers from Allyl Methacrylate
J. P. J. HIGGINS and K. E. WEALE,
Department of Chemical Engineering and Chemical Technology,
Imperial College of Science and Technology, London, England
synopsis
Allyl methacrylate has been polymerized by free-radical methods and found to yield
a soluble polymer in carbon tetrachloride, dioxane, and diallyl ether solutions. The
l,s.
overall rate equation in diallyl ether is R , = k [ln]o.7[M] It is suggested that propagation and cyclization reactions proceed only via addition to the methacrylyl groups of
the monomer. Some degradative chain transfer occurs with the allyl groups, and it is
considered that the solvents may ensure the production of soluble polymers by reactions
in which allyl-radical side chains are terminated without crosslinking.
INTRODUCTION
It has been shown‘ that 1,6-dienes can, under certain conditions, yield
soluble polymers by an alternating inter-intramolecular mechanism. Previous attempts to obtain a soluble polymer from the monomer allyl metha ~ r y l a t ehave, however, not been successful. Kawai3has attributed this
~,~
to the predominance of the intermolecular reaction and to degradative
chain transfer .
Allyl methacrylate (I), which is an unsymmetrical 1,6-diene, has now
been found to produce soluble polymers up to high conversions in solutions
of carbon tetrachloride (CClJ, dioxane, and diallyl ether. Examination
of the polymers so produced shows that they are true homopolymers and
that in diallyl ether little or no copolymerization occurs with the solvent.
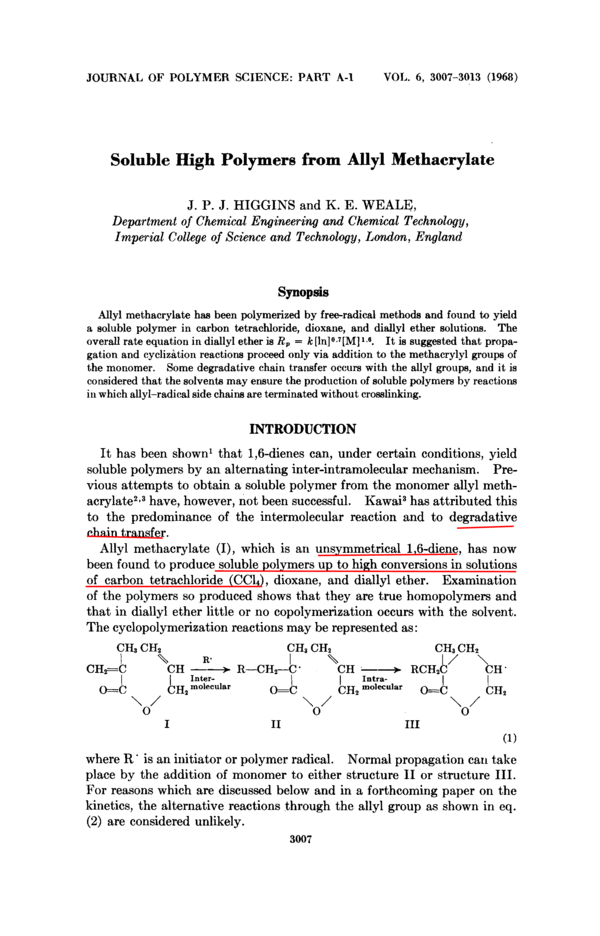
The cyclopolymerization reactions may be represented as :
CHs CHz
CHFC I
\CH
I
I
R
+
R-CHz-b.
CHa CHz
\CH
I
I
Inter-
CH, molecular
O=C
I
O=C
Intramolecular
CHa CHz
RCHZC/
I
\cH.
I
I
I1
‘’
0
CH,
0
‘
’
O=C
CHz
‘/
O
where R ‘ is an initiator or polymer radical. Normal propagation can take
place by the addition of monomer to either structure I1 or structure 111.
For reasons which are discussed below and in a forthcoming paper on the
kinetics, the alternative reactions through the allyl group as shown in eq.
(2) are considered unlikely.
3007
3008
J. P. J. HIGGINS AND K. E. WEALE
IV
V
EXPERIMENTAL
The monomer and all of the solvents were obtained from standard commercial sources and were distilled under vacuum before use. Benzoyl
peroxide (Bz202)
was recrystallized from chloroform solution. Polymerizations were carried out in sealed glass ampules in an oil bath at 6O"C, and
the rate of polymerization R, was determined gravimetrically. Monomer
and diallyl ether which were exposed to air for a few days turned yellow, presumably through absorption of oxygen, and these samples gave relatively
low yields when used in polymerizations. However, a few samples prepared by the high vacuum filling technique gave results similar to those
obtained from freshly distilled monomer and solvent, but with no special
precautions to exclude air. Infrared absorption spectra of the monomer
and polymer were obtained on a Hilger H-800 spectrometer with an NaCl
prism.
RESULTS
Ally1 methacrylate (M) was polymerized in bulk and in solution in
toluene, benzene, acetone, CCL, isopropyl ether, epoxypropane, dioxane,
and diallyl ether, with benzoyl peroxide initiator. With the reactions
in bulk, and in the solvents toluene, benzene, acetone, isopropyl ether,
and epoxypropane, gelation occurred at very low conversions. The polymers were insoluble in the common organic solvents, in the monomer, and
in dimethyl sulfoxide. I n CC1, solution, at low concentrations, conversions of up to 40% soluble polymer were obtained before gelation occurred.
Autoacceleration was also observed and careful purification techniques and
the exclusion of oxygen did not prevent its occurrence. I n dioxane solutions, a t concentrations of up to 2.2 mole/l monomer, high yields of soluble polymer were obtained. I n diallyl ether solution very high yields of
soluble polymer were also obtained. The gel point occurred at lower conversions as the concentration of the monomer was increased. At very low
monomer concentrations almost 100% soluble polymer was obtained.
Figure 1 shows the results for the polymerization in three solvents a t fixed
monomer and initiator concentrations.
In various attempts to homopolymerize dioxane and diallyl ether by
free-radical methods4a t temperatures from 30°C to 100°C and a t pressures
from 1 to loo00 atm. it was found that these substances would not polymerize, (diallyl ether is, however, polymerized by cationic catalysts such as
boron trifluoride diethyl etherate). Elemental analyses of the polymers
SOLUBLE HIGH POLYMERS
3009
60
50
1
20
10
I
0
1
2
3
I
4
5
I
1
7
6
a
TIME (hrs)
Fig. 1. Polymerization of allyl methacrylate in three solvents at 60°C: (a) dioxane; ( b )
diallylether; (c) CC14. [BzzOz] = 1.1 X 10+ mole/l; [MI = 1 mole/l.
of allyl methacrylate formed in dioxane and diallyl ether solutions confirmed
that little or no copolymerization had occurred and that the allyl methacrylate had formed homopolymers. Table I shows the effect on the rate of
polymerization of allyl methacrylate in diallyl ether solution as the initiator concentration is varied at constant monomer concentration.
TABLE I
Polymerization of Ally1 Methacrylate at 60°Ca
No.
[BzzOZ] lo3
X
molefl
X 106,
moleflaec
R p
~
1
2
3
4
5
1.03
5.80
16.80
27.20
45.20
1.01
3.04
5.16
8.49
12.40
* Solvent: diallyl ether; [MI : 2.2 mole/l.
The plot of log R , versus log [Bzz02Jis linear and has a slope of 0.7.
Table I1 shows the effect on the rate of polymerization of allyl methacrylate in diallyl ether solution as the monomer concentration is varied at
constant initiator concentration.
The plot of log R , versus log [M J is linear and has a slope of 1.6. Thus
the rate of polymerization of allyl methacrylate in diallyl ether solution at
G0"C in the range of concentrations studied is given by:
R , = k[B~202]O.'[M]~.'
(3)
J. P. J. HIGGINS AND K. E. WEALE
3010
TABLE I1
Polymerization of Ally1 Methacrylate at 60°C.a
RP X 10:
[MI,
mole/l
mole/l-sec
1.1
1.8
2.2
3.3
3.6
4.4
6.1
No.
27.7
57.6
84.9
172.7
176.9
282.2
580.1
* Solvent: diallyl ether; [Bz201] 1.1 x
:
lo-* mole/l.
where k is a constant. Such irregular orders with respect to initiator and
monomer concentrations are common for allylic and substituted allylic
monomers.
Characterization of Polymer
Intrinsic viscosity was measured in an Ubbelohde viscometer a t 25°C
in toluene solution. The intrinsic viscosity [v] varied from 0.1 to 0.5.
Because of residual unsaturation the intrinsic viscosity varied with the
amount of time the polymer had been exposed to air, and all polymer samples so exposed eventually became insoluble due to crosslinking. Using
freeze-dried polymer samples which were immediately dissolved in toluene
it was possible to obtain an intrinsic viscosity-molecular weight relationship by comparing intrinsic viscosity results with osmometrically determined molecular weights for the same samples. The Mark-HouwinkSakurada equation for poly(ally1 methacrylate) in toluene at 25°C was
found to be:
= 2.4
x
10-4AT~0.65
(4)
kfnis the number-average molecular weight. In the case of poly(ally1
methacrylate), formed in diallyl ether solution a t 60°C using BzzOz as the
initiator, the molecular weight varied from 70000 to 1 O O .
OOO
Infrared Spectra
The infrared spectra of the monomer and the polymer are given in
Figure 2.
In the spectrum of the monomer the carbonyl stretch frequency absorption exhibits a peak a t 1725 cm-' but in the polymer this peak is broadened
and shifted towards 1740 cm-'. This indicates the presence of Glactone
units (six-membered rings) in the polymer. The spectrum of the polymer
also shows a small absorption at 1775 cm-l. This is attributed to the
presence of a small proportion of -y-lactone units (five-membered rings)
which may be formed as shown in eq. (5).
SOLUBLE HIGH POLYMERS
F
R4Hz
‘
I
O=C
3011
CHs
CH=CHz
I
+
RCHzC-CH-CHz’
o=b
CHz
I
\’
O
I1
(5)
&HZ
\’
O
VI
The presence of two types of lactone ring in the polymer is supported
by the absorptions a t 1230 and 1275 cm-’. These are probably due to the
-COstretching frequencies in the 6- and y-lactone rings.
WAVE NUHBW (--I)
Fig. 2. Infrared spectra of (-)
allyl methacrylate and (- -) poly(ally1 methacrylate)
at 8W1800 em-’.
DISCUSSION
It has long been known that allylic monomers undergo degradative chain
transfer (DCT) reactions.6.’ In these reactions, which compete with normal propagation, a hydrogen atom is extracted from the monomer by an
active radical. In the case of allyl acetate:
R-
+ C H ~ C H C H ~ O C C H RH + CH~=CHCHOCCH~
~
-+
II
0
It
(6)
0
The resulting acetoxyallyl radical is resonance-stabilized and does not
readily add monomer, although it may terminate by bimolecular combination. If DCT predominates the rate of polymerization varies approximately as the first power of the initiator concentration6 rather than the 0.5
power found with normal monomers such as styrene and methyl methacrylate. The results for allyl methacrylate show that R , varies as the 0.7
power of the initiator concentration. This result may be explained if the
methacrylyl groups can add to chains and undergo cyclizationwith the allyl
groups as shown in eq. (l), while the allyl groups preferentially undergo
f
DCT rather than the reactions shown in eq. (2). I the allyl group is
3012
J. P. J. HIGGINS AND K. E. WEALE
pendant on a polymer or radical chain, which is denoted by X, the DCT
reaction is:
The pendant resonance-stabilized allyl radicals do not propagate further
with monomer but may terminate by bimolecular combination in two
ways :
2 VII
* CH-CH4H-X
AH*
I
VIII
CH
AH
I
X‘
2 VII
+
X-CH=CHCHzCHzCH=CH-X’
IX
(9)
When there are many pendant resonance-stabilized allyl radicals these
reactions would quickly lead to crosslinked gel polymers and may account
for the formation of insoluble polymers in polymerization in bulk and in
the solvents toluene, benzene, acetone, isopropyl ether, and epoxypropane.
The formation of soluble polymers in the other solvents suggests that in
these the resonance-stabilized allyl radicals are preferentially terminated
by a process which does not lead to crosslinking. Carbon tetrachloride
is an active chain-transfer agent in some polymerizations8~9
and ethers
and dioxane are known to cause rapid induced decomposition of benzoyl
peroxide.lOpll The allyl groups of the solvent diallyl ether can also take
part in DCT reactions. Radical-solvent and radical-initiator reactions
wl produce small rapidly diffusing radicals which would be expected to
i
l
react with pendant resonance-stabilized allyl radicals much more rapidly
than the latter can combine mutually. The differences between the polymer obtained in the two groups of solvents may thus be explicable in
terms of the differing abilities of the solvents to bring about termination
of radical side chains before they can react.
It has been shown that methacrylyl groups are much more reactive than
allyl groups in the polymerizations of the methacrylic ester of 2-allylphenol12 and allyl methacrylate.la The polymerization proceeds via the
methacrylyl groups to give high molecular weight polymer. That fraction
of the allyl groups not included in cyclization reactions via the methacrylyl
groups are left pendant on the growing chains.
On the view that the allyl group does not enter into chain addition
reactions it is possible to make some simplifications in the kinetic equations
for the polymerization of allyl methacrylate, and these are discussed in a
forthcoming paper.
SOLUBLE HIGH POLYMERS
3013
References
1. G. B. Butler and R. J. Angelo, J. Amer. Chem. SOC.,
79,3128 (1957).
2. S. G. Cohen, B. E. Ostberg, and D. B. Sparrow, J. Polym. Sci.,3,264 (1948).
3. W. Kawai, J. Polym. Sn'. A-l,4,1191 (1961).
4. J. P. J. Higgins and K. E. Weale, unpublished results.
5. P. D. Bartlett and R. Altachul, J. Amer. Chem. SOC.,
67,812,816 (1945).
6. R. Hart and G. Smets, J. Polym. Sci.,
5,55 (1950).
7. P. D. Bartlett and F. A. Tate, J. Amer. Chem. SOC.,
75.91 (1953).
8. R. A. Gregg and F. R. Mayo, J. Amer. Chem. SOC.,
70,2373 (1948).
9. S. Palit and S. K. Das, Proc. Roy. SOC.
(London), A226.82 (1954).
10. P. D. Bartlett and K. Nozaki, J . Amer. Chem. SOC.,
69,2299 (1947).
11. W. E. Cass, J . A m . Chem. SOC.,
69,500 (1947).
12. 0. F. Solomon, M. G. Corviovei, and V. Tarareacu, J. Appl. Polym. Sn'., 11,1631
(1967).
13. S. Cohen, B. E. Ostberg, D. B. Sparrow, and E. R. Blount, J. Polym. Sn'., 3,264
(1948).
14. J. P. J. Higgins and K. E. Weale, to be published.
Received January 4,1968
Revised March 19, 1968
Coments go here:
- Log in to post comments